Neuroscience is often complicated by the inability to precisely study function in live subjects; even the best methods of imaging are often insufficient to examine detailed events occurring within brains. Therefore, posthumous dissection and evaluation of model organisms (e.g. mice, flies) is the norm. However, this usually comes with a steep monetary cost. Moreover, due to the complexity of the human brain, random signals from around the brain make searching for relevant data like finding a needle in a haystack.
Brain organoids are a developing branch of organoid-engineering, or the development of organ-mimicking groups of cells, and aim to solve the problem of complicated signals of neuroscience research. Usually, brain organoids develop from stem cells retrieved from the neural ectoderm, a particular region of embryonic tissue from where much of the central nervous system grows. The cells are grown in vitro (i.e. in a cultured dish) with a designed scaffold.[1] Using specialized methods, certain glial cells (support cells found in the central nervous system) can also be integrated into the organoid.[2]
A potential use of brain organoids is in the study of neurodegenerative diseases. Precursor stem cells can be genetically modified, so brain organoids can be grown with careful genetic mutations to study the genetic causes of neurodegenerative diseases like Alzheimer’s.[5] In addition, they can be used as 3-D models of complex illness, which are often reduced to 2-D study in brain slices.[6]
Furthermore, brain organoids offer a clearer understanding of neurodevelopmental disorders. Naturally, the study of brain development is limited due to the difficulty and cost of experimenting and imaging a developing embryo. As such, brain organoids function as a strong substitute, since researchers can use them to look closely at early development of the brain.[6] For example, early forms of brain organoids were an integral part of the study of the Zika virus.[7]
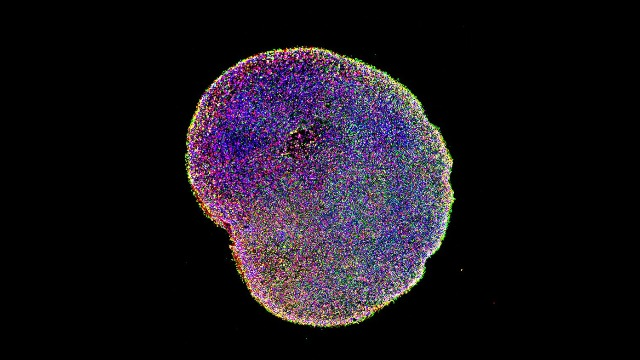
An slide of a fluorescently-labeled cerebral organoid
Source: Paola Arlotta laboratory, Harvard University, retrieved from https://www.technologynetworks.com/neuroscience/news/brain-organoid-boost-replicates-human-cerebral-cortex-growth-320303
However, the use of brain organoids has limitations. As seen above, these organoids lack the intricate folds, or gyri, characteristic of human brains. Indeed, neural organoids lack many of the common features associated with normal brains; they do not have the ability to use senses (since no other organs are connected), nor the ability to feel temperature or pain. As a result, the higher-level use of brain organoids is limited by their relative simplicity.[2]
One significant limitation to developing more complex neural organoids has been the inability to supply adequate nutrients to larger organoids. Complex organoids provide more accurate models for study, but without blood vessels on the inside of these solid blobs, tissue in the center dies.[3] The rise of 3-D-printing technology has allowed for human-generated vascular construction in organoids,[3] which has major implications not just on neural organoid construction, but on tissue engineering as a whole. Furthermore, pseudo-vascularized systems have recently been developed.[4] Combined, these new technologies pave the way for better neural organoids that can advance the study of the brain and its development.
Some evidence does suggest that these brain organoids can function on the level of a second-trimester embryo, and can exhibit oscillating brain wave patterns.[8] Thus, an ethical problem emerges. As developments in engineering brain organoids continue, they will become increasingly realistic and reflective of real brains; consciousness and cognition could potentially arise, not on purpose, but as a byproduct of development.[9] Furthermore, without measurable outward signals, it would be difficult to determine the extent of such consciousness, complicating evaluation.[10]
Brain organoids can theoretically be classified as either living human tissue or experimental tissue culture. Each of these classifications poses unique ethical questions. Since the consciousness of every-day humans resides wholly within the brain as far as modern research knows, then a living brain, despite being developed in a lab, would also in theory be considered fully human. Thus, if advanced brain organoids could be considered human, then conducting research using them would necessitate preliminary trials using models of other animals, compounding cost for limited gains.
On the other hand, since brain organoids are just one organ, sustained by careful engineering of nutrients and vascular growth, it is difficult to accept the advanced organoid as an independently living organism rather than just an advanced form of tissue culture. Since the evaluation and origin of consciousness is currently unclear, formally considering a brain organoid as “conscious”, given that sensory input is non-existent, is quite ambiguous. Researchers will be confronted with the question of the risk of testing on a conscious brain versus the benefits that research may produce. In case-by-case analysis, the ethical quandaries become vague and difficult to evaluate.
Given the benefits and potential ethical risks of brain organoids, the future of development must occur with great caution, and with great involvement of bioethicists. Though fully developed brain organoids are far from reality, research will increasingly fall under scrutiny and it is up to the public to decide on the ethical implications of such research.
References:
- Lancaster, M., Renner, M., Martin, C. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013). https://doi.org/10.1038/nature12517
- Koo, B., Choi, B., Park, H., & Yoon, K. J. (2019). Past, Present, and Future of Brain Organoid Technology. Molecules and cells, 42(9), 617–627. https://doi.org/10.14348/molcells.2019.0162
- Grebenyuk, S., & Ranga, A. (2019). Engineering Organoid Vascularization. Frontiers in bioengineering and biotechnology, 7, 39. https://doi.org/10.3389/fbioe.2019.00039
- Cakir, B., Xiang, Y., Tanaka, Y. et al. Engineering of human brain organoids with a functional vascular-like system. Nat Methods 16, 1169–1175 (2019). https://doi.org/10.1038/s41592-019-0586-5
- Seo J, Kritskiy O, Watson LA, et al. Inhibition of p25/Cdk5 Attenuates Tauopathy in Mouse and iPSC Models of Frontotemporal Dementia. J Neurosci. 2017;37(41):9917-9924. doi:10.1523/JNEUROSCI.0621-17.2017
- Jeong, H., Jimenez, Z., Mukhambetiyar, K. et al. Engineering Human Brain Organoids: From Basic Research to Tissue Regeneration. Tissue Eng Regen Med (2020). https://doi.org/10.1007/s13770-020-00250-y
- Qian X, Nguyen HN, Song MM, et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 2016;165(5):1238-1254. doi:10.1016/j.cell.2016.04.032
- Trujillo CA, Gao R, Negraes PD, et al. Complex Oscillatory Waves Emerging from Cortical Organoids Model Early Human Brain Network Development. Cell Stem Cell. 2019;25(4):558-569.e7. doi:10.1016/j.stem.2019.08.002
- Lavazza A, Massimini MCerebral organoids: ethical issues and consciousness assessmentJournal of Medical Ethics 2018;44:606-610.
- Cheshire, William P,J.R., M.D. CEREBRAL ORGANOIDS AND THE THRESHOLD OF CONSCIOUSNESS. Ethics & Medicine. 2020;36(1):27. http://ezproxy.cul.columbia.edu/login?url=https://search-proquest-com.ezproxy.cul.columbia.edu/docview/2390570101?accountid=10226.
