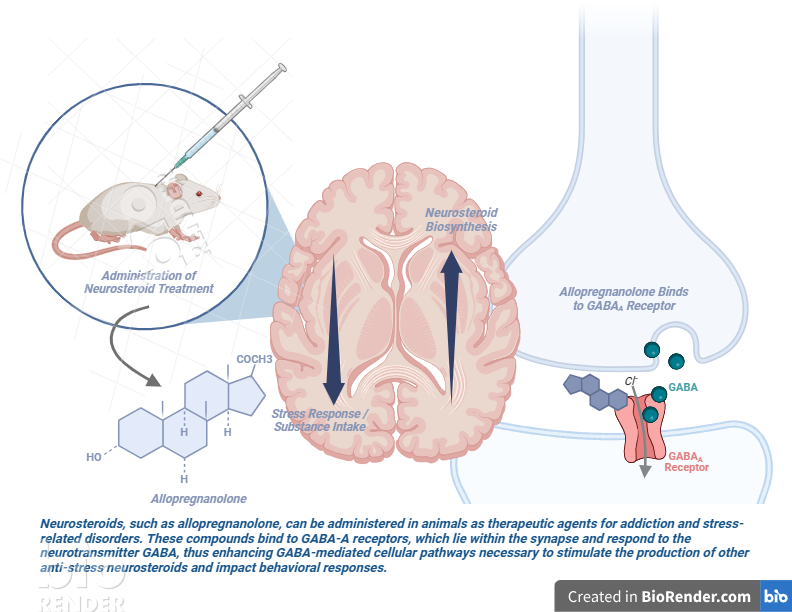
From alcohol addiction to anxiety, psychological illnesses are pervasive in today’s society. While scientists have long struggled to find effective and sustainable treatment methods, pharmacologists are now pointing to neurosteroids—the brain’s natural stress relievers—as potential therapeutic agents against neuropsychiatric disorders.
Synthesized primarily in the central nervous system as a response to stress, neurosteroids are fast-acting modulators of brain excitability; in other words, they alter the sensitivity of neurons through binding to their membrane receptors. Neurosteroids primarily interact with specific sites on GABAA receptors, which are cell membrane proteins that control the brain’s exchange of chemical messengers, or neurotransmitters. Naturally occurring neurosteroids, like allopregnanolone and tetrahydrodeoxycorticosterone (more commonly known as THDOC), work to enhance GABAA-mediated pathways, helping compounds that are important for inducing anti-stress effects to bind to the receptor.
For pharmacologists, such neurosteroids show huge promise in their anticonvulsant, anti-anxiety, memory enhancing, and sedative-hypnotic properties (Reddy, 2010). As with any kind of biomedical research, however, scientists face challenges in translating findings from in vivo to in vitro and applying animal models to human studies. Among these are accounting for hormone and sex differences in the nervous system and compensating for the poor water solubility of neurosteroids, which impact the feasibility of clinical studies. Nevertheless, neuropharmacology research has gained significant momentum. In recent years, a variety of lab-manufactured neurosteroids have been engineered, with applications ranging from veterinary anesthesia to epilepsy and substance abuse treatments.
It has long been established that alcohol targets the GABAA receptor by mimicking its neurotransmitter counterpart GABA and inducing GABA’s cascade effects, thereby increasing the production of neurosteroids while impairing both cognition and motor coordination. Alcohol dependence and withdrawal ultimately come with an increased risk of seizures; thus, researchers investigated how administering neurosteroids to alcohol-withdrawn rats at various doses might change their susceptibility to such seizures (Janis et al., 1998). The study found that neurosteroid treatments given just before the experimental rats’ seizure thresholds protected them from the increased seizure susceptibility compared to control rats’ seizure thresholds. Building off of these findings, another study investigated how stimulating neurosteroid production led to changes in cocaine self-administration in addicted rats (Schmoutz et al., 2015). Using treatments of oxazepam and metyrapone, diagnostic compounds that block the synthesis of pro-stress steroids, researchers then extracted and quantified levels of neurosteroids allopregnanolone and THDOC from the rat brain tissue samples. Their findings demonstrated that the treatments increased the production of anti-stress neurosteroids in brain regions that are relevant to drug addiction, thus suppressing cocaine-directed behavioral responses. As research continues, the stimulation of neurosteroid biosynthesis proves effective in reducing substance intake and dependence.
Beyond addiction, the use of neurosteroids as potential therapeutics may extend to attention hyperactivity deficit disorder (ADHD), anxiety, and depression. A recent study found that neurosteroids play an important role in mitigating stress in pregnant guinea pigs (Crombie et al., 2021). Psychosocial stress during pregnancy has been associated with increased neurodevelopmental and emotional issues, as well as reduced neuron production, in young children. In this study, pregnant guinea pigs were exposed to stress-inducing strobe lights. Pups from the experimental group were then postnatally administered doses of neurosteroid ganaxolone and emapunil, an anti-stress drug that stimulates the production of other neurosteroids, before having their total neurons and neurosteroids quantified. The postnatal treatments led to sex-dependent differences in hyperactivity and development of mature neurons due to a positive feedback loop that increased neurosteroid concentrations relative to levels in the control group. Researchers hypothesize that such neurosteroid-based treatments promote corrective processes in early development, leading to improved behavioral outcomes. While they have yet to determine why there were sex-based differences in the efficacy of neurosteroid treatments, scientists have thus established the therapeutic potential of neurosteroids for stress-related disorders.
Recently, the direction of neurosteroid research has expanded towards treating epilepsy, acute anxiety disorders, premenstrual syndrome, and even Alzheimer’s disease, with numerous therapeutics companies racing to provide solutions to these conditions. Though there still remain hurdles to cross, additional findings on neurosteroid-based therapies may be the key to opening new doors in the future of pharmacology.
References
Crombie, G. K., Palliser, H. K., Shaw, J. C., Hodgson, D. M., Walker, D. W., & Hirst, J. J. (2021). Neurosteroid-based intervention using Ganaxolone and Emapunil for improving stress-induced myelination deficits and neurobehavioural disorders. Psychoneuroendocrinology, 133, 105423. https://doi.org/10.1016/j.psyneuen.2021.105423
Finn, D. A., Roberts, A. J., & Crabbe, J. C. (1995). Neuroactive Steroid Sensitivity in Withdrawal Seizure-Prone and -Resistant Mice. Alcoholism: Clinical and Experimental Research, 19(2), 410–415. https://doi.org/10.1111/j.1530-0277.1995.tb01523.x
Gatta, E., Camussi, D., Auta, J., Guidotti, A., & Pandey, S. C. (2022). Neurosteroids (allopregnanolone) and alcohol use disorder: From mechanisms to potential pharmacotherapy. Pharmacology & Therapeutics, 240, 108299. https://doi.org/10.1016/j.pharmthera.2022.108299
Janis, G. C., Devaud, L. L., Mitsuyama, H., & Morrow, A. L. (1998). Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3alpha-Hydroxy-5alpha-pregnan-20-one in male and female rats. Alcoholism: Clinical and Experimental Research, 22(9), 2055. https://doi.org/10.1097/00000374-199812000-00023
Morrow, A. L., VanDoren, M. J., Penland, S., & Matthews, D. B. (2001). The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Research Reviews, 37(1–3), 98–109. https://doi.org/10.1016/s0165-0173(01)00127-8
Reddy, D. S. (2010). Neurosteroids. In Elsevier eBooks (pp. 113–137). https://doi.org/10.1016/b978-0-444-53630-3.00008-7
Schmoutz, C. D., Guerin, G. F., Runyon, S. P., Dhungana, S., & Goeders, N. E. (2015). A therapeutic combination of metyrapone and oxazepam increases brain levels of GABA-active neurosteroids and decreases cocaine self-administration in male rats. Behavioural Brain Research, 291, 108–111. https://doi.org/10.1016/j.bbr.2015.05.019
Zorumski, C. F., Mennerick, S., & Izumi, Y. (2014). Acute and chronic effects of ethanol on learning-related synaptic plasticity. Alcohol, 48(1), 1–17. https://doi.org/10.1016/j.alcohol.2013.09.045
