At the heart of all living beings lies the central dogma of biology. This fundamental process orchestrates the flow of genetic information from DNA to RNA to proteins, which carry out all of the vital functions of the cell—from structure to metabolism. This shared genetic blueprint also allows organisms to acquire genes from one another. Scientists have leveraged this power to manufacture key medicines like insulin or to produce biofuels, all by manipulating the genes of bacteria (Khan et al., 2016). However, this universal genetic code also leaves cells vulnerable to the attack of viruses. These invaders cunningly hijack the cell’s machinery by directing the replication of their own DNA, creating rampant contamination. After decades of effort, researchers recently achieved a remarkable breakthrough that produced a strain of bacteria resistant to viruses, enhancing the safety and efficiency of these genetically engineered bacteria.
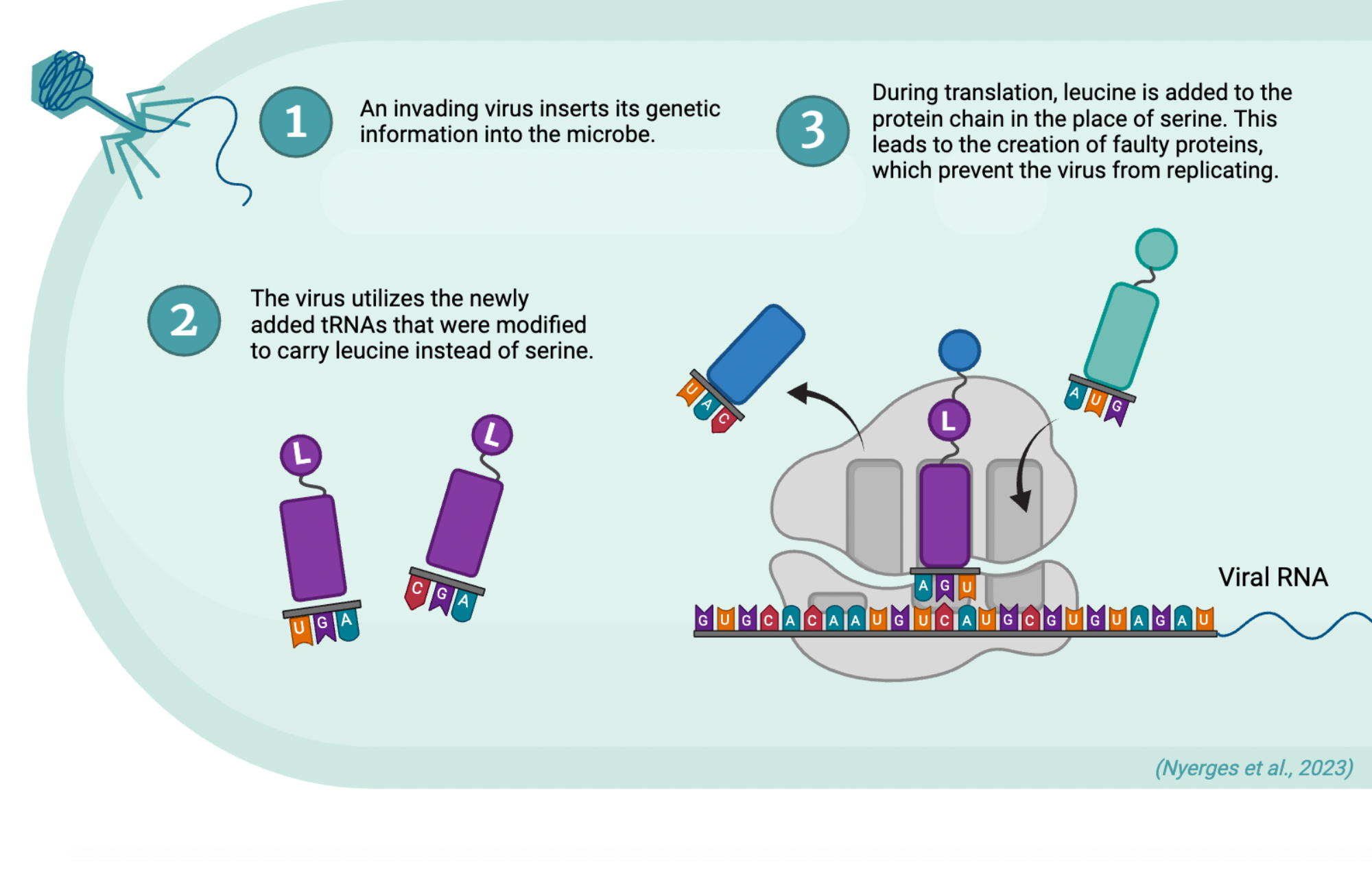
This breakthrough traces back to 2004, when synthetic biologist George Church developed an intriguing theory. During translation, the process in which RNA sequences are read into protein strands, certain transfer RNA (tRNA) molecules recognize corresponding triple-nucleotide sequences that are called codons. These codons help tRNA molecules bring the corresponding building blocks for protein synthesis: amino acids, such as leucine and serine. With 64 codons but only 20 amino acids to code for, Church questioned the necessity of the extra codons and considered their removal. He hypothesized that deleting these codons would create dead ends for viruses attempting to hijack host cell machinery as the corresponding tRNA would no longer exist, thereby preventing the production of viable viral proteins during replication.
At the time, Church’s hypothesis was a mere fantasy; it was difficult enough to alter a single gene in an organism, let alone edit the thousands of genes necessary to remove every instance of a specific codon. Many years later, Church finally put his ideas to the test. In 2013, his lab at Harvard Medical School made a small modification to a stop codon in the E. coli genome and altered the tRNA so that when it recognized the original stop codon in the genome of an invading virus, it installed the wrong amino acid (Lajoie et al., 2013). This study was the first to demonstrate that a bacterium was able to produce its own proteins but was immune to viruses.
In a recent Nature study, Church's team, led by post-doc Akos Nyerges, showcased a novel E. coli strain that exhibited resistance to all tested molecular invaders (Nyerges et al., 2023). This work was built upon a previous study conducted at the University of Cambridge a year earlier, where researchers replaced a stop codon and two serine codons in E. coli, aiming to prevent viral hijacking by removing the tRNAs recognizing the original serine codons (Zürcher et al., 2022). However, despite these edits, Nyerges and his colleagues discovered that the modified E. coli remained susceptible to infection when confronted with various viruses (Nyerges et al., 2022).
Nyerges and Church quickly identified the problem: simply deleting codons was insufficient as some viruses could get around the missing codons by using their own viral tRNAs to help themselves replicate. These scientists realized they had to develop a method to change what those codons tell an organism to make. Using multiplex gene-editing technology, the Church Lab removed the two serine codons along with their tRNAs, which were replaced by new tRNAs that carry leucine instead of serine. These modified tRNAs can overpower the viruses’ own tRNAs, leading to faulty viral proteins. Compared to their initial attempt almost a decade ago, this E. coli strain showed resistance to all tested molecular invaders, serving as a simple one-step method to turn any organism virus-proof.
These findings could ultimately lower the risk of viral contamination in factories where bacteria are used to produce important pharmaceuticals or biofuels, saving millions of dollars. In addition, the team incorporated a synthetic amino acid into the microbe to add a layer of safety, ensuring these genetically engineered bacteria would perish if they escaped the lab environment. While some researchers still question whether these microbes are fully virus-resistant, we have yet to see an invader break this firewall.
References
Khan, S., Ullah, M. W., Siddique, R., Nabi, G., Manan, S., Yousaf, M., & Hou, H. (2016). Role
of recombinant DNA technology to improve life. International Journal of Genomics, 2016, 1–14. https://doi.org/10.1155/2016/2405954
Lajoie, M. J., Rovner, A. J., Goodman, D. B., Aerni, H.-R., Haimovich, A. D., Kuznetsov, G.,
Mercer, J. A., Wang, H. H., Carr, P. A., Mosberg, J. A., Rohland, N., Schultz, P. G., Jacobson, J. M., Rinehart, J., Church, G. M., & Isaacs, F. J. (2013). Genomically recoded organisms expand biological functions. Science, 342(6156), 357–360. https://doi.org/10.1126/science.1241459
Nyerges, A., Vinke, S., Flynn, R., Owen, S. V., Rand, E. A., Budnik, B., Keen, E., Narasimhan,
K., Marchand, J. A., Baas-Thomas, M., Liu, M., Chen, K., Chiappino-Pepe, A., Hu, F., Baym, M., & Church, G. M. (2022). Swapped genetic code blocks viral infections and gene transfer. bioRxiv. https://doi.org/10.1101/2022.07.08.499367
Nyerges, A., Vinke, S., Flynn, R., Owen, S. V., Rand, E. A., Budnik, B., Keen, E., Narasimhan,
K., Marchand, J. A., Baas-Thomas, M., Liu, M., Chen, K., Chiappino-Pepe, A., Hu, F., Baym, M., & Church, G. M. (2023). A swapped genetic code prevents viral infections and gene transfer. Nature, 615(7953), 720–727. https://doi.org/10.1038/s41586-023-05824-z
Zürcher, J. F., Robertson, W. E., Kappes, T., Petris, G., Elliott, T. S., Salmond, G. P., & Chin, J. (2022). Refactored genetic codes enable bidirectional genetic isolation. Science, 378(6619), 516–523. https://doi.org/10.1126/science.add8943
