Tick. Tock. Kidney.
Tick. Tock. Liver.
Tick. Lung.
Tock. Heart.
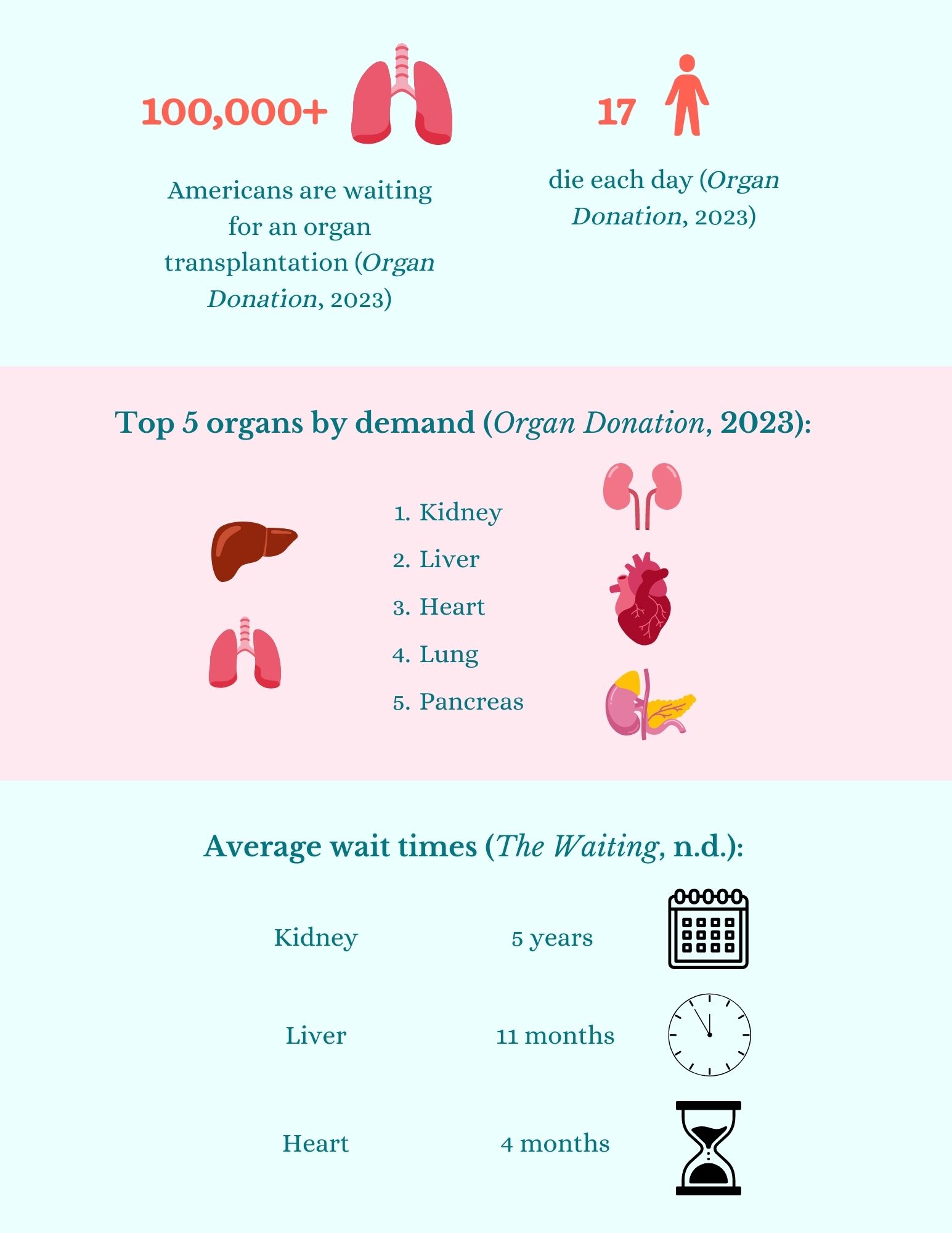
Tick. Seventeen people die every day waiting for an organ transplant in the US. Over 100,000 people are currently on the national organ transplant waiting list (Organ Donation, 2023).
Tock.
Several obstacles stand in the way of successful organ transplants. Ethical principles govern the practice of accepting organs that have been donated rather than sold, yet organ trafficking and illegal transplants continue. Moreover, there is a shortage of organs and not enough donors to save everyone in need of a transplant. Some organs are even rejected by the recipients’ immune systems and must be removed (Beyar, 2011). Organs cannot be stored indefinitely; their viability outside the donor is limited to only 4 hours, depending on the organ (Scudellari, 2015). Consequently, some people who need transplants end up losing hope.
However, there is an unlikely source of hope: plants. Plant scaffolding is an experimental method where cells are removed from different parts of plants, such as the leaves and stem. Various processes, including a sodium dodecyl sulfate (also known as detergent) bath, are used to create space for other cells to grow. The scaffold retains its shape because of a hard material called cellulose from plant cell walls, thus providing a structure and vasculature for the new cells (Harris et al., 2021).
The 3-dimensional nature of these plant scaffolds allows cells to grow in a lab environment that is closer to the human body than a glass dish. Plant vasculature involves a series of tubelike tissues that transport water and nutrients, which is similar to the blood vessels in human organs. Additionally, cellulose has low immunogenicity, meaning it elicits a weak immune response. It is biocompatible and cannot be degraded within mammals due to the absence of cellulase enzymes, therefore it might be safely implanted without adverse effects. Many plant scaffolds are also coated with other materials like fibronectin, a molecule found on the outside of cells, to help the new cells adhere to the scaffold and grow (Harris et al., 2021).
Because of these properties, scientists have attempted to build human organs from plant scaffolds. Researchers successfully seeded spinach leaves with heart muscle cells, which were able to contract as if they were in a beating heart (Gershlak et al., 2017). Bone cells have been grown in apples (Lee et al., 2019), skeletal muscle cells have grown and matured in green onions (Cheng et al., 2020), and even ears have been grown from apple scaffolds (Eng, 2016). How would you like to sport a pair of apple ears?
Organs have not been fully recreated from plant scaffolds yet, but smaller scale transplantations have been accomplished. For instance, one study used an apple scaffold to grow bone cells. Rats with skull defects received the scaffolds, leading to bone healing (Zhu et al., 2021). Another study implanted apple scaffolds under the skin of mice to study immune response. The scaffolds were later removed, and they retained their shape while eliciting low inflammation in the mice (Harris et al., 2021). There have even been attempts to model the pancreas’s insulin production.
While current plant scaffolds might not be able to save the thousands of patients waiting for a new organ in the U.S., they do indicate the potential for continued research into the possibility of scaffolds to create artificial organs (Organ Donation, 2023). The cost efficiency and availability of plants make them ideal candidates for organ transplants. Even patients who are not waiting for a transplant, such as people with type 1 diabetes whose pancreatic islets fail to produce enough insulin, could benefit from the production of these plant scaffold organs. Let’s look forward to a brighter future, one where plants can save our lives!
References
Beyar, R. (2011). Challenges in organ transplantation. Rambam Maimonides Medical Journal, 2(2). https://doi.org/10.5041/rmmj.10049
Cheng, Y.-W., Shiwarski, D. J., Ball, R. L., Whitehead, K. A., & Feinberg, A. W. (2020). Engineering aligned skeletal muscle tissue using decellularized plant-derived scaffolds. ACS Biomaterials Science & Engineering, 6(5), 3046-3054. https://doi.org/10.1021/acsbiomaterials.0c00058
Eng, K. F. (2016, February 16). A promising way to grow body parts … using an apple. Ideas.TED.com. Retrieved August 1, 2023, from https://ideas.ted.com/a-promising-way-to-grow-body-parts-using-an-apple/
Gershlak, J. R., Hernandez, S., Fontana, G., Perreault, L. R., Hansen, K. J., Larson, S. A., Binder, B. Y.k., Dolivo, D. M., Yang, T., Dominko, T., Rolle, M. W., Weathers, P. J., Medina-bolivar, F., Cramer, C. L., Murphy, W. L., & Gaudette, G. R. (2017). Crossing kingdoms: Using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials, 125, 13-22. https://doi.org/10.1016/j.biomaterials.2017.02.011
Harris, A. F., Lacombe, J., & Zenhausern, F. (2021). The emerging role of decellularized plant-based scaffolds as a new biomaterial. International Journal of Molecular Sciences, 22(22), 12347. https://doi.org/10.3390/ijms222212347
Lee, J., Jung, H., Park, N., Park, S.-H., & Ju, J. H. (2019). Induced osteogenesis in plants decellularized scaffolds. Scientific Reports, 9(1). https://doi.org/10.1038/s41598-019-56651-0
Organ donation statistics. (2023, March). Health Resources & Services Administration. Retrieved August 1, 2023, from https://www.organdonor.gov/learn/organ-donation-statistics
Scudellari, M. (2015, March 4). U.S. funds efforts to freeze human organs for long-term storage. Scientific American. Retrieved August 1, 2023, from https://www.scientificamerican.com/article/u-s-funds-efforts-to-freeze-human-organs-for-long-term-storage/
The waiting list. (n.d.). Gift of Life Donor Program. Retrieved August 1, 2023, from https://www.donors1.org/patients/resources-for-transplant-patients/the-waiting-list/
Zhu, Y., Zhang, Q., Wang, S., Zhang, J., Fan, S., & Lin, X. (2021). Current advances in the development of decellularized plant extracellular matrix. Frontiers in Bioengineering and Biotechnology, 9. https://doi.org/10.3389/fbioe.2021.712262
