In May 2022, 13-year-old patient Alyssa had already tried all conventional treatments for her Leukemia but had no success. Now, more than one year later, Alyssa shows no signs of cancer. How is this possible? Well, it is all thanks to the world’s first “base editing” therapy.
What is base editing, and how does it work?
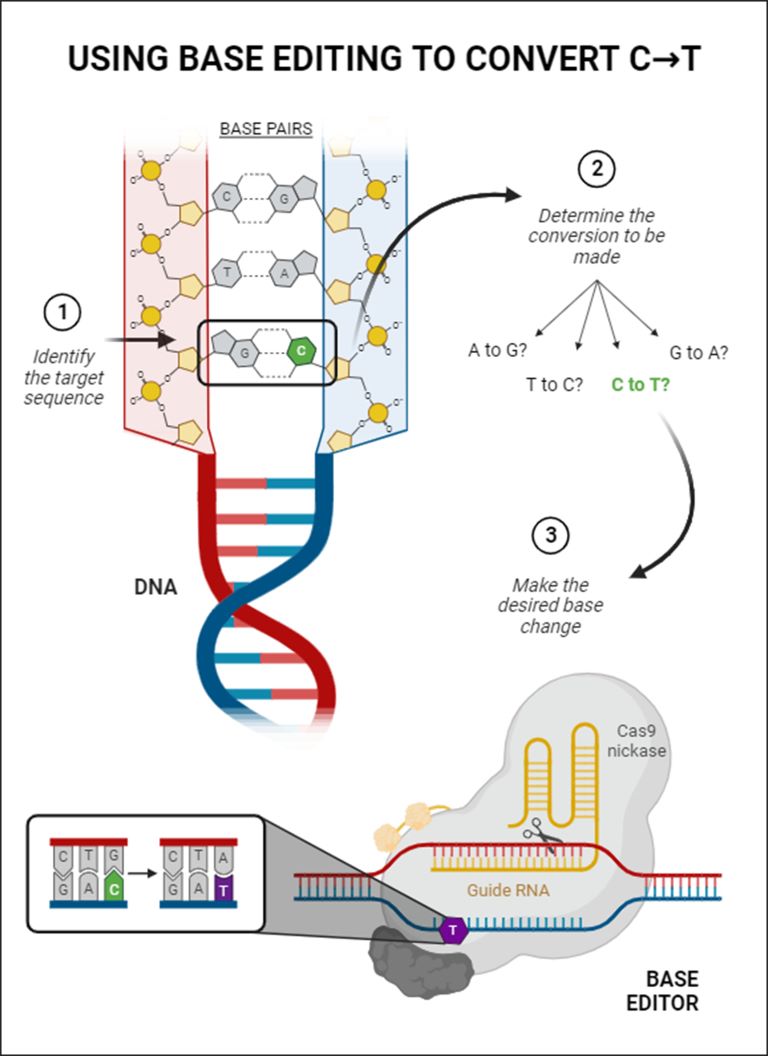
Our biological information lies in sequences of DNA bases, which are found in pairs that bind together the two DNA strands. There are four types of bases: cytosine (C), thymine (T), guanine (G), and adenine (A). Just a single base mutation in the DNA sequence can be responsible for a genetic disease. In fact, Dr. David Liu (2021) affirms that "the conversion of As to Gs and Ts to Cs would correct about half of known pathogenic single-letter mistakes."
In response to this problem, a team of researchers led by Dr. Liu at the Broad Institute of MIT and Harvard developed base editing: technology that corrects DNA misspellings by converting one base into another (Komor et al., 2016). Specifically, there are Cytosine base editors (CBEs) and Adenine base editors (ABEs); together, they support 4 base changes (C→T, G→A, T→C, & A→G).
The base editing process starts with a disabled CRISPR/Cas9 Protein: A Cas9 nickase (Cas9 enzyme mutation) and a guide RNA work to find the target DNA sequence, where the Cas9 nickase creates a single-strand DNA break. Then, a base-converting enzyme makes the desired base change, and the cell repairs itself. Through this final repair, the base edit becomes permanent (Liu, 2023).
What is base editing’s current status?
In recent years, base editing has been tested in mice with progeria, a genetic disorder resulting from a single C-to-T base mutation (Koblan, 2021). But, one of the latest developments in base editing therapy is the case of Alyssa, a cancer patient at the UK’s Great Ormond Street Hospital for Children.
Alyssa was diagnosed with T-cell Acute Lymphoblastic Leukemia (T-ALL), an aggressive and uncommon subtype of Acute Lymphoblastic Leukemia (ALL) that attacks a type of white blood cell called T lymphocytes or T cells. Accounting for 12-15% of all ALL cases, T-ALL is an uncommon kind of Leukemia, and there are few treatments for it (Raetz & Teachey, 2016).
Sadly, neither chemotherapy nor a bone marrow transplant worked in clearing Alyssa’s cancer. Fortunately, in May 2022, Alyssa and her family opted into a revolutionary yet experimental base-editing therapy, thus becoming "the first patient to be treated with this technology" (Qasim, 2022).
What did Alyssa's revolutionary treatment consist of?
To treat Alyssa, researchers worked with healthy donor T cells, which were engineered to have a CD7-specific chimeric antigen receptor (CAR) (Chiesa et al, 2023). A CAR can program a T cell to find and attack cells with a particular antigen, a marker recognized by the immune system (Sterner & Sterner, 2021). In Alyssa’s treatment, the CAR programmed the donor cells to find and fight T cells, which express the CD7 antigen (Chiesa et al., 2023; UCL, 2023).
Both Alyssa’s malignant cells and the donor cells were T cells, meaning that the two of them had the same CD7 marker. As a consequence, the engineered donor T cells would find and attack not only the cancerous cells but also each other (UCL, 2023). To prevent this, scientists used base editing to inactivate the CD7 marker of the donor cells by converting C→T, stopping the production of the CD7 protein and, thus, inactivating the CD7 marker (Chiesa et al, 2023). As a result, the donor CAR T cells only attacked Alyssa’s cancerous T cells–not each other.
Additionally, scientists made some extra edits to the donor cells 1) to protect them against any ongoing cancer treatment and 2) to avoid the graft-versus-host disease (when donor cells see the healthy patient cells as foreign and attack them) (Chiesa et al, 2023). After these edits, the donor CAR T cells were administered to Alyssa, showing positive results (Qasim, 2022).
What will be the future of human gene editing?
Scientists consider “Prime editing” as the next generation of precision gene-editing tools (Wang et al., 2022). Prime editors support 12 base-to-base conversions and, additionally, correct base insertions and deletions (Anzalone et al., 2019; Liu, 2021). Certainly, the number of edits within prime editing surpasses that within base editing. This would allow scientists to find treatments or cures for a broader range of diseases – if considering only the benefits to gene editing, that is.
It is important to acknowledge the downsides to this technology, especially when questioning its future. The National Heart, Lung, and Blood Institute states that using genome editing in humans could have potential consequences such as “certain types of cancer, allergic reactions, or damage to organs or tissues if an injection is involved” (NHLBI, 2022). After all, Alyssa’s experimental therapy is the first time base editing has been used in humans, meaning that there is still much to know about the risks of using the present and future generations of gene-editing technologies.
Moreover, one of the most prevalent arguments against the alteration of human genomes is that it could be misused. Indeed, a mistakenly edited gene could result in a heritable genetic disease, and what if this detrimental edit was done on purpose (Radcliff, 2017)? For concerns like this, governments are creating guidelines to use gene editors. An example of this is the European Oviedo Convention, which “specifically outlaws heritable genome editing” (Coller, 2019).
While the future is unpredictable, one thing is clear: we must remain hopeful that, whether through gene editing or through some other revolutionary therapy, scientists will give a chance at life to millions of patients around the world, just like they gave it to Alyssa.
References
Anzalone, A.V., Randolph, P.B., Davis, J.R. et al. (2019). Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157. doi:10.1038/s41586-019-1711-4
Chiesa, R., Georgiadis, C., Syed, F. et al. (2023). Base-Edited CAR7 T Cells for Relapsed T-Cell Acute Lymphoblastic Leukemia. The New England Journal of Medicine. doi:10.1056/NEJMoa2300709
Coller, B. S. (2019). Ethics of human genome editing. Annual Review of Medicine, 70, 289-305. doi:10.1146/annurev-med-112717-094629
Gaudelli, N., Komor, A., Rees, H. et al. (2017). Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551, 464–471. doi:10.1038/nature24644
Koblan, L.W., Erdos, M.R., Wilson, C. et al. (2021). In vivo base editing rescues Hutchinson–Gilford progeria syndrome in mice. Nature 589, 608–614. doi:10.1038/s41586-020-03086-7
Komor, A., Kim, Y., Packer, M. et al. (2016). Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424. doi:10.1038/nature17946
Liu, D. (Host). (2023, April 19). David Liu at Imagine Solutions 2023 [Video]. YouTube. The Conversation. https://www.youtube.com/watch?v=0XLdz4e_ML4&list=WL&index=5
Liu, D. (Guest). (2021, Jun 7). The Future of Genome Editing with Professor David Liu. (No. 99) [Audio podcast episode]. In The FYI – The For Your Information Podcast. ARK Invest. https://ark-invest.com/podcast/ep-99-david-liu/
National Heart, Lung, and Blood Institute (NHLBI). (2022, March 24). Genetic Therapies - Benefits and Risks. National Institutes of Health. https://www.nhlbi.nih.gov/health/genetic-therapies/benefits-risks#:~:text=Genetic%20therapies%20hold%20promise%20to,if%20an%20injection%20is%20involved
Qasim, W. (2022, December 21). Alyssa’s story: Base Editing & CAR T-Cell Therapy at Great Ormond Street Hospital [Video]. YouTube. Great Ormond Street Hospital and Charity (GOSH). https://www.youtube.com/watch?v=x4clNXVVLJw
Radcliff, S. (2017, August 26). Will Gene Editing Allow Us to Rid the World of Diseases? Healthline. https://www.healthline.com/health-news/will-gene-editing-allow-us-to-rid-world-of-diseases
Raetz, E.A., Teachey, D.T. (2016). T-cell acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program; 2016 (1): 580–588. DOI:10.1182/asheducation-2016.1.580
Sterner, R.C., & Sterner, R.M. (2021). CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69. doi: 10.1038/s41408-021-00459-7
University College London (UCL). (2023, June 15). Further hope for base-edited T-cell therapy to treat resistant leukaemia. UCL News. https://www.ucl.ac.uk/news/2023/jun/further-hope-base-edited-t-cell-therapy-treat-resistant-leukaemia
Wang D, Fan X, Li M, Liu T, Lu P, Wang G, Li Y, Han J, Zhao J. Prime Editing in Mammals: The Next Generation of Precision Genome Editing. CRISPR J. 2022 Dec;5(6):746-768. doi: 10.1089/crispr.2022.0084. PMID: 36512351.
