Target therapy is a medical treatment that targets certain disease-causing molecules, genes, or cells and has fewer side effects than other medicines. With cancer being the second-leading cause of death in the United States, drug resistance in cancer cells treated with target therapy stands as a grave barrier to achieving long-term success (American Cancer Association, 2022). Researchers have discovered the culprits behind this inconvenient phenomenon: extrachromosomal DNAs (ecDNAs) and the non-homologous end joining (NHEJ) pathway (Winstead, 2023). Together, they make once-effective drugs ineffective at fighting cancer; since target therapies serve as the common treatment for most cancers, their failure leaves patients with few treatment options. However, new research published in the American Association for Cancer Research in April 2023 titled “Blocking Genomic Instability Prevents Acquired Resistance to MAPK Inhibitor Therapy in Melanoma” has perhaps found a way to block cells from gaining resistance. Before diving into this research, gaining an understanding of ecDNAs and the NHEJ pathway is necessary.
ecDNAs are circular pieces of DNA that have split off from chromosomes, which can lead to changes in the genome because they contain additional replicas of genes that control cell growth and division. When ecDNAs replicate within the body, it results in a heightened number of replicas of these genes within the cells that can misbalance the regular agents of cell growth and division, which play an essential role in cancer development. ecDNAs are classified by size as either a small type or a large type. The large types of ecDNAs are only located in cancer cells and hold cancer-activating genes. Furthermore, ecDNAs are associated with the enhancer region, which allows for the rapid replication of cells within the body, resulting in high concentrations in multiple cancers (Wang, 2022). ecDNAs result in cells gaining resistance to drugs used in target therapies because they are unstable in gene amplification (Verhaak, Bafna, Mischel, 2019). Combining gene amplification and genes that control cell development results in a variety within cancer cells. Over time, the resistance gained by cancer cells to target therapy leads to the treatment failing. Thus, the high levels of ecDNAs found in cancers are correlated with shorter survival rates for patients.
A DNA double-strand break (DSB) is DNA damage that occurs when both sides of the DNA double helix are broken (Mourad, Ginalski, Legube, and Cuvier, 2018). DSB can occur for multiple reasons, including radiation, ultraviolet light (UV), and chemicals (Mourad, Ginalski, Legube, and Cuvier, 2018). It is also widely considered the most dangerous form of DNA damage; if not corrected, DSB leads cells to malfunction (Cox, 2013). There are two forms of repairing a DSB: recombinational DNA repair and non-homologous DNA end-joining (NHEJ). Recombinational DNA repair tends to be accurate, whereas NHEJ usually adds or deletes genetic information (Cox, 2013). Despite NHEJ playing an important role in repairing DSB, it also plays an important role in cancer development. Cancer is primarily developed due to changes in genome activity, so the mutations that occur as a result of the added or deleted genetic information caused by the NHEJ pathway can result in an increase in cancer frequency.
Moving on to the study, scientists discovered that skin cancer cells, specifically melanoma cells, depend on the NHEJ pathway to produce replicas of genes that may be tied to drug resistance (Winstead, 2023). Researchers used cells from melanoma that contained genetic changes that result in uncontrolled cell growth which is either BRAF V600 or NRAS Q61. Researchers studied the genes after the melanoma cancer cells were treated with target therapy; they repeated this until the cells stopped reacting to the treatment. Furthermore, they discovered that the cancer cells underwent a rearrangement of chromosomes leading to genetic changes, otherwise known as chromothripsis, leading to ecDNAs. Since ecDNAs are linked to enhancer components, this results in rapid replication of the cells that have genetic changes.
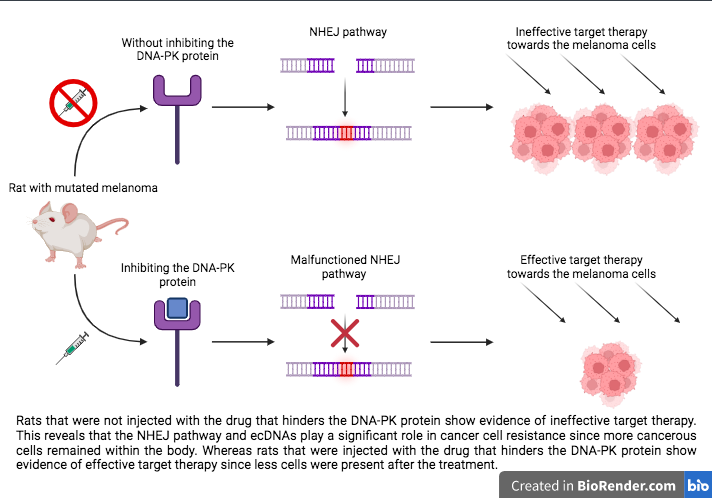
Afterwards, scientists moved onto blocking the NHEJ pathway testing in mice. They used a drug that hinders the DNA-PK protein, which is crucial to the function of the NHEJ pathway within DSB because it identifies any breaks in DNA. Mice were injected with melanoma cancer cells containing the BRAF V600 or NRAS Q61 mutations. The hindered DNA-PK protein resulted in a delay or prevention of the cells developing resistance to the targeted therapy.
As of now, there are clinical trials in the works to test the hindered DNA-PK protein in human patients with melanoma. If the results from the clinical trial are similar, then it has the ability to stop cancerous cells from developing resistance, which, in turn, will allow patients to not seek other treatment options.
Ultimately, this study on ecDNAs and the NHEJ pathway offers hope for fighting cancer cell resistance. The results are hopeful, however cautious optimism is needed. There’s a longing for effective cancer treatment, and these clinical trials might be the hope people are looking for.
References
2022 Cancer Facts & Figures Cancer | Cancer Death Rate Drops. (2022, January 12). Www.cancer.org. https://www.cancer.org/research/acs-research-news/facts-and-figures-2022.html#:~:text=Cancer%20continues%20to%20be%20the
Davis, A. J., & Chen, D. J. (2013). DNA Double Strand Break Repair via non-homologous end-joining. Translational Cancer Research, 2(3), 130–143. https://doi.org/10.3978/j.issn.2218-676X.2013.04.02
Dharanipragada, P., Zhang, X., Liu, S., Lomeli, S. H., Hong, A., Wang, Y., Yang, Z., Lo, K. Z., Vega-Crespo, A., Ribas, A., Moschos, S. J., Moriceau, G., & Lo, R. S. (2023). Blocking Genomic Instability Prevents Acquired Resistance to MAPK Inhibitor Therapy in Melanoma. Cancer Discovery, 13(4), 880–909. https://doi.org/10.1158/2159-8290.cd-22-0787
Dr Susan Lees-Miller. (2015). What is the DNA-dependent protein kinase, DNA-PK? - ecancer. In ecancer.org. https://ecancer.org/en/video/4574-what-is-the-dna-dependent-protein-kinase-dna-pk
Extrachromosomal DNA | Cancer Grand Challenges. (n.d.). Cancergrandchallenges.org. https://cancergrandchallenges.org/challenges/extrachromosomal-dna
Mao, Z., Bozzella, M., Seluanov, A., & Gorbunova, V. (2008). DNA repair by nonhomologous end joining and homologous recombination during cell cycle in human cells. Cell Cycle, 7(18), 2902–2906. https://doi.org/10.4161/cc.7.18.6679
Mourad, R., Ginalski, K., Legube, G., & Cuvier, O. (2018). Predicting double-strand DNA breaks using epigenome marks or DNA at kilobase resolution. Genome Biology, 19(1). https://doi.org/10.1186/s13059-018-1411-7
Non-homologous End-joining Pathway - Creative Diagnostics. (n.d.). Www.creative-Diagnostics.com. https://www.creative-diagnostics.com/non-homologous-end-joining-pathway.htm
Stinson, B. M., & Loparo, J. J. (2021). Repair of DNA Double-Strand Breaks by the Nonhomologous End Joining Pathway. Annual Review of Biochemistry, 90(1). https://doi.org/10.1146/annurev-biochem-080320-110356
Wang, P. (2022, April 20). ecDNAs are Extra Active and Terrifying - NCI. Www.cancer.gov. https://www.cancer.gov/ccg/blog/2022/interview-ecdna
Winstead, E. (2023, March 17). Preventing Resistance to Cancer Targeted Therapies - NCI. Www.cancer.gov. https://www.cancer.gov/news-events/cancer-currents-blog/2023/preventing-resistance-cancer-targeted-therapies
Yi, E., Chamorro González, R., Henssen, A. G., & Verhaak, R. G. W. (2022). Extrachromosomal DNA amplifications in cancer. Nature Reviews Genetics, 23(12), 760–771. https://doi.org/10.1038/s41576-022-00521-5
