Whenever I find myself at a fast food drive-through, my mind returns to a picture from an infamous experiment, where a McDonald’s hamburger is left out for several years and, by some mysterious concoction of preservatives, remains intact. I would joke with friends that the burger was made out of ‘Franken-meat’ created in a lab, hence its ability to defy natural decomposition. Of course, we did not believe the meat was manufactured in a lab. The mere possibility of lab-grown meat seemed too much like science fiction.
Nowadays, however, what was once considered unfathomable has entered the realm of reality. This past June, the USDA approved two companies, UPSIDE Foods and GOOD Meat, to begin producing the first cell-cultured chicken—that is, meat synthesized in a lab to have the same biochemical make-up and cellular profile as meat obtained from a living chicken.
The implications for bringing cultivated meat to the market are significant, as doing so would provide a means to satisfy the growing demand for meat while simultaneously offering potential solutions to urgent societal issues; for example, cultivated meat could possibly lower carbon emissions attributable to meat manufacturing, reduce animal suffering, and minimize the risk for zoonotic disease outbreaks (Ching et al., 2022).
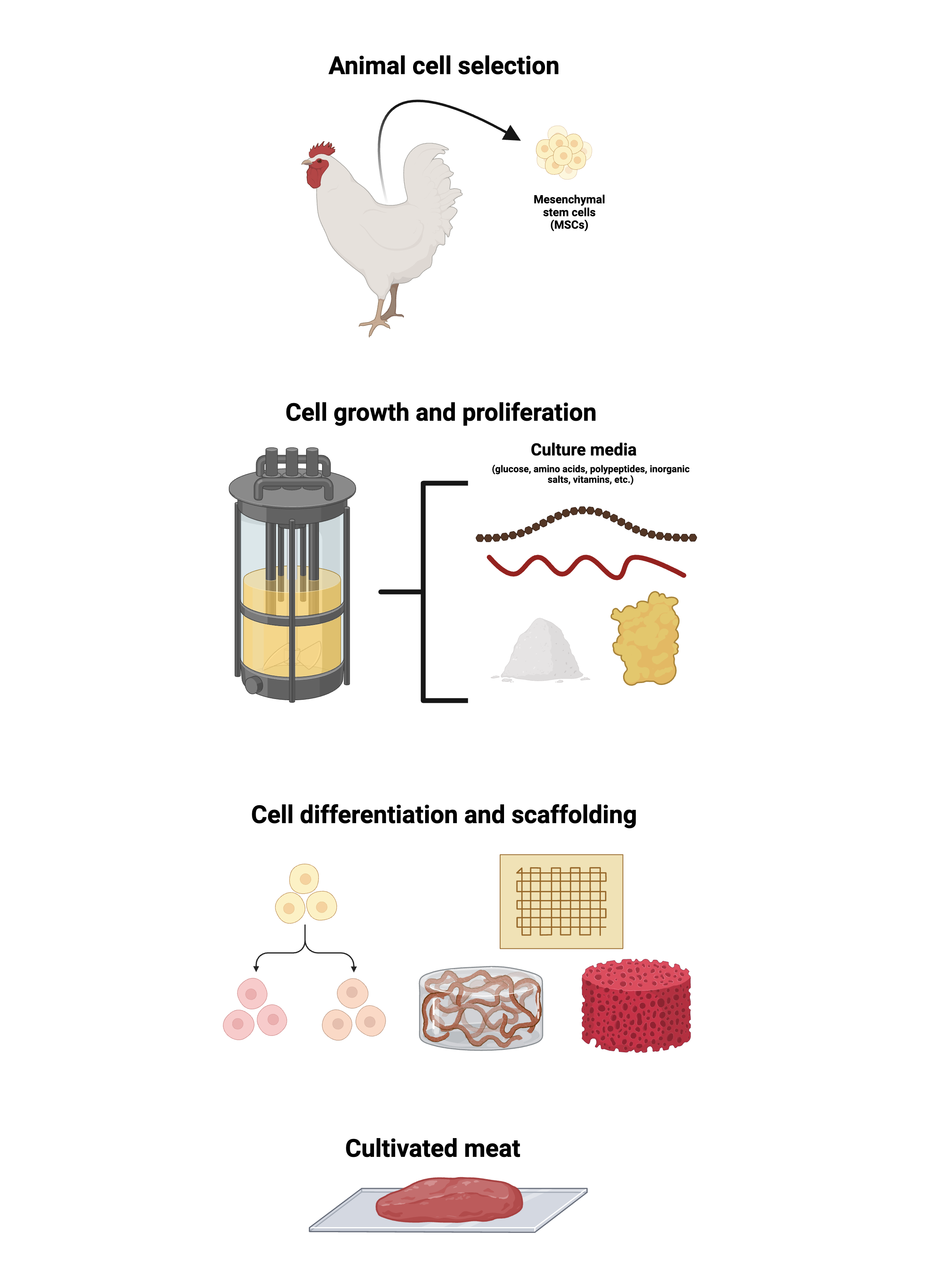
Understandably, though, there exists a stigma around lab-grown meat. Consumers are skeptical about eating meat procured from somewhere other than a live animal. That said, cultivated meat is still technically real meat. To understand why and how, a demystification of the production process is warranted. There are three primary stages in the manufacturing of cultivated meat: animal cell selection, cell growth and proliferation, and scaffolded cell maturation to form an edible end-product (Reiss et al., 2021).
Animal Cell Selection
To begin the process of creating meat, a sample from the animal is obtained, and a subset of cells taken from this sample is allowed to multiply. These cells are termed progenitor cells. Progenitor cells are special because they descend from stem cells, known for possessing the property of multipotency. A cell that is multipotent can change its structure and function through the process of differentiation.
The type of progenitor cells isolated from the sample are mesenchymal stem cells (MSCs), which are sourced from an animal’s skeletal muscles (Knežić et al., 2022). MSCs are especially relevant because they can differentiate into adipocytes, osteoblasts, and chondrocytes—all types of cells found in meat (Choi et al., 2020; Klimczak et al., 2018; Schwartz, 2023).
Cell Growth and Proliferation
The type of cell that a group of MSCs becomes is partially dependent on the type of substance the cells are ‘fed’ to grow on (Schwartz, 2023). During this stage, the progenitor cells are placed in a bioreactor, which is a tool that can be used to isolate and support specific chemical reactions to produce a desired product.
Within the bioreactors, the cells are exposed to a growth medium that consists of glucose, amino acids, inorganic salts, vitamins, and other components necessary for animal cell growth. As the MSCs develop and multiply, they also begin to differentiate into their final cellular identity.
Cell Differentiation and Scaffolding
Similar to the scaffolds you see when walking by buildings under construction, cells rely on a structural framework to guide their development. Most cells, including MSCs, require attachment to a physical surface before they start developing (Merten, 2015). This characteristic is referred to as anchorage-dependency.
Materials that can be used for scaffolding vary in their biochemical compositions, with cellulose (carbohydrate), lignin (fibrous complex that includes carbohydrates), and zein (protein) being some popular materials used currently (Swartz, 2023a). This material must also contain oxygen for the developing cells. Through a series of biochemical processes, the scaffold directs the cells to a place to grow and differentiate relative to other cells in space. Like the choice of cell culture media, the choice of scaffold material can influence the final identity of the cell (Swartz, 2023a).
The Future of Cultivated Meat: Is This So-called ‘Franken-Meat’ Here to Stay?
After being shown a preview of the cultivated meat manufacturing process, you may be wondering how feasible it would be to scale up production enough to make a difference in the lives of consumers. In 2023, the net cost for producing one pound of cultivated meat is still substantial, with a recent techno-economic analysis (TEA) estimating it to be $29.50 (Quint, 2023). Even so, given that the first cultivated meat burger was created a decade ago for over $300,000, the industry’s prospects look more optimistic (Fountain, 2013). Considering this progress within the context of the worsening climate crisis, the ongoing inhumane treatment of livestock, and the spread of zoonotic illnesses, it is not unreasonable to predict that cultivated meat could soon be a viable alternative to conventional meat products (Hayek, M.N., 2022; Tuomisto & Teixeira de Mattos, 2011). Given another ten years of research and development in the cultivated meat sector, perhaps the fast food burger you get at the drive-through may contain meat sourced not from the farm, but from the lab.
References
Ching, L., Zainal, N., Luang-In,V., Ma, N. (December 2022). Lab-based meat the future food. Environmental Advances, 10. https://doi.org/10.1016/j.envadv.2022.100315
Choi, K., Yoon, J.W., Lee, H.J., Jeong, J., Ryu, M., Jo, C., Lee, C. (6 November 2020). Muscle stem cell isolation and in vitro culture for meat production: A methodological review. Comprehensive Reviews in Food Science and Food Safety, 20(1), p. 429-457.
Fountain, H. (2013, May 12). Engineering the $325,000 In Vitro Burger. The New York Times. https://www.nytimes.com/2013/05/14/science/engineering-the-325000-in-vitro-burger.html
Klimczak, A., Kozlowska, U., Kurpisz, M. (2018). Muscle Stem/Progenitor Cells and Mesenchymal Stem Cells of Bone Marrow Origin for Skeletal Muscle Regeneration in Muscular Dystrophies. Archivum Immunologiae et Therapiae Experimentalis, 66, p. 341-354. doi: 10.3390/biom12050699
Knežić, T., Janjušević, L., Djisalov, M., Yodmuang, S. Gadjanski, I. (13 May, 2022). Using Vertebrate Stem and Progenitor Cells for Cellular Agriculate, State-of-the-Art, Challenges, and Future Perspectives. Biomolecules, 12(5), p. 699. doi: 10.3390/biom12050699
Merten, O. (5 February, 2015). Advances in cell culture: anchorage dependence. Philosophical Transactions of the Royal Society: Biological Sciences, 370(1661). doi:10.1098/rstb.2014.0040
Quint, Y. (11 July, 2023). Cultivated Meat’s Path to Price Parity. Ark. https://www.ark-biotech.com/post/cultivated-meats-path-to-price-parity
Reiss, J., Robertson, S., Suzuki, M. (22 July 2021). Cell Sources for Cultivated Meat: Applications and Considerations throughout the Production Workflow
Schwartz, E. (2021). Deep Dive: Cultivated meat cell culture media. The Good Food Institute. https://gfi.org/science/the-science-of-cultivated-meat/deep-dive-cultivated-meat-cell-culture-media/
Swartz, E. (2023). Deep dive: Cultivated meat cell lines. The Good Food Institute. https://gfi.org/science/the-science-of-cultivated-meat/deep-dive-cultivated-meat-cell-lines/
Swartz, E. (2023a). Deep dive: Cultivated meat scaffolding. The Good Food Institute. doi: https://gfi.org/science/the-science-of-cultivated-meat/deep-dive-cultivated-meat-scaffolding/
Tuomisto, H.L., Teixeira de Mattos, M.J.T. (17 June, 2011). Environmental impacts of cultured meat production. Environmental Science & Technology. doi: 10.1021/es200130u
