What are periods? No, really. What is it that comprises the monthly bleeding that half of the population experiences?
It should come as no surprise that approximately 1.8 billion people menstruate every month worldwide (Rohatgi & Dash, 2023). However, less is widely known—and discussed—about the biological components of periods. Menstrual blood is composed of three distinct body fluids: blood, vaginal fluid, and the shedding of the uterine lining during menstruation. But for scientists, that same menstrual blood may also be the next cutting-edge tool in regenerative medicine. Researchers believe in its wide-ranging capability to treat degenerative conditions such as Alzheimer’s disease and to repair damaged or deteriorated organs such as the heart and skin.
The discovery of menstrual blood-derived stem cells by Xiaolong Meng et al. in 2007 rattled the field of regenerative medicine. Stem cell therapy, a form of regenerative medicine, aims to repair injured or diseased cells using lab-grown stem cells, which are manipulated to develop into specialized cells such as nerve and cardiac cells. For example, for a person with heart disease, stem cells could be injected into heart muscle to differentiate into cardiac cells, subsequently repairing the injured tissue.
While stem-cell therapy holds promise in alleviating suffering and paving the way for groundbreaking treatments, there are a host of challenges and ethical considerations associated with conventional methods of cell acquisition.
In stem cell therapy, researchers conventionally use embryonic or adult stem cells. Embryonic stem cells, which have the ability to differentiate into all cell types, are derived from the inner cell mass of early-stage embryos, resulting in their destruction (Faramarzi et al., 2016). On the contrary, adult stem cells are commonly derived from mesenchymal stem cells—a type of adult stem cell—of bone marrow and umbilical cord blood, all of which involve invasive procedures (Faramarzi et al., 2016). Adult stem cells have restricted differentiation potency, indicated by their ability to differentiate into cell types within a limited lineage (Faramarzi et al., 2016). A limited lineage means that adult stem cells can follow a restricted path of differentiation to become only certain types of cells.
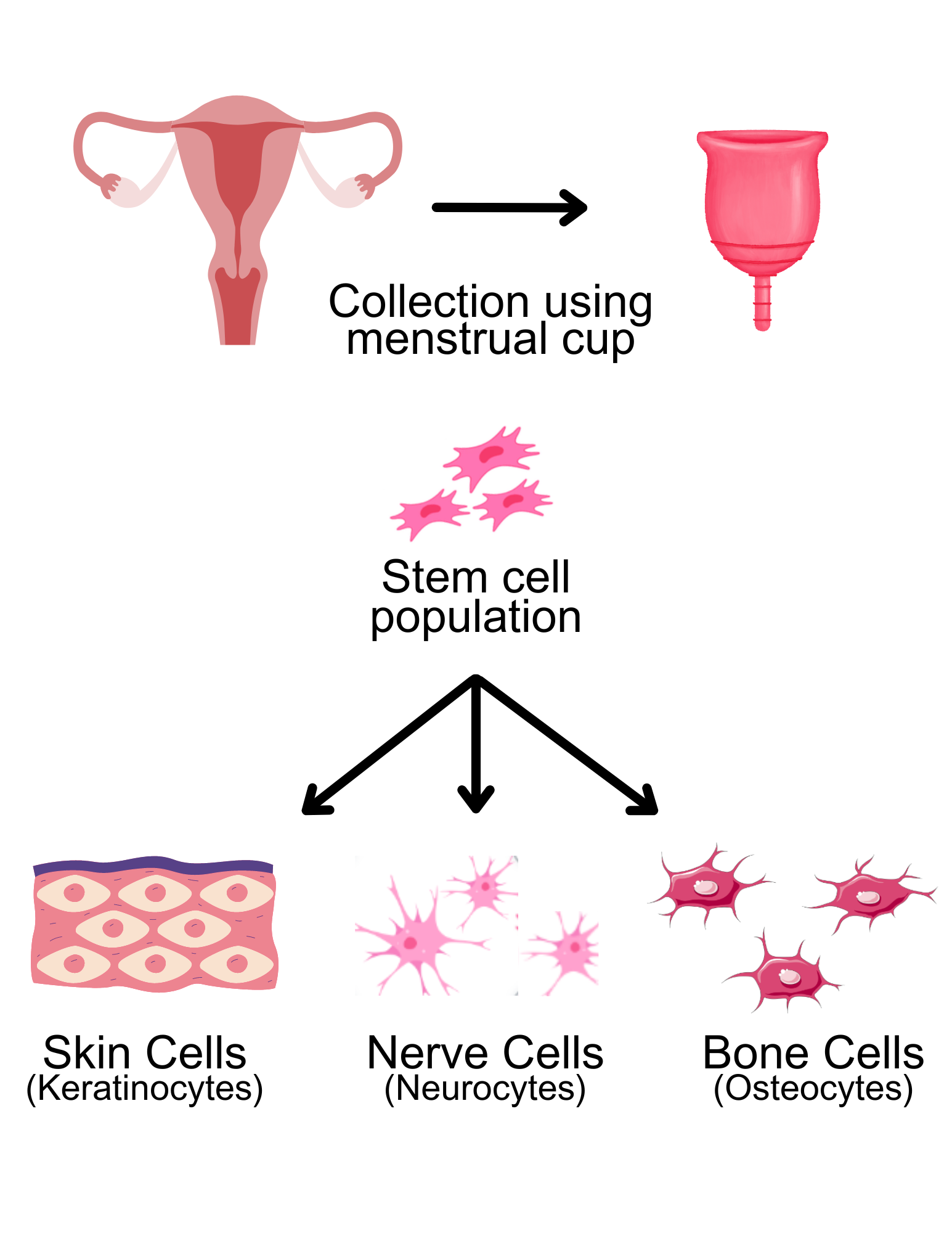
Contrary to the stem cells obtained invasively, menstrual blood-derived stem cells can be harvested by simply collecting the monthly shedding of the endometrium. Menstrual blood-derived stem cells eliminate many associated ethical concerns and the need for invasive operations for acquiring of stem cells. But most of all, menstrual-blood derived stem cells stand out in their accessibility. However, this begs the question: how viable are stem cells derived from menstrual blood?
Research indicates that menstrual blood-derived stem cells express a range of characteristics similar to that of both embryonic and adult stem cells, indicated by specific cell markers in certain conditions (Faramarzi et al., 2016). As opposed to adult stem cells derived from bone marrow, menstrual blood-derived stem cells possess the capacity to undergo multi-lineage differentiation, allowing for a greater range in their treatment capabilities. Further research has also found that menstrual blood-derived stem cells are able to differentiate into cells in bone, fat, cartilage formation, liver, and cardiac muscle, to varying capacities (Sayeh Khanjani et al., 2014). Such versatility in treatment capabilities holds considerable promise for potential therapeutic applications in the future.
Expanding on this potential, a recent study conducted by Yanling Zhang et al. simulated the use of menstrual blood-derived stem cells to treat intrauterine adhesions, a condition resulting in scar-tissue buildup in the uterus, within mice (Zhang et al., 2016). Researchers treated the mice using menstrual blood derived-stem cells, taken from human menstrual blood, which were injected into the mice through their tails (Zhang et al., 2016). The injected cells were able to migrate to the injury site and transform into cells within the uterine lining (Zhang et al., 2016). Following the treatment, the mice made an incredible recovery, showing significant improvement in their uterus lining and fertility (Zhang et al., 2016). Zhang’s finding suggests that menstrual blood-derived stem cell therapy could be the next method of treatment for intrauterine adhesions in humans.
Menstrual blood-derived stem cells have also demonstrated effectiveness in treating an array of other diseases in mouse models. These diseases include stroke, Type 1 diabetes, acute and chronic liver diseases, acute lung injury, epithelial ovarian cancer, Alzheimer’s disease, heart diseases, and neurodegenerative diseases, among others (Chen, Qu, Cheng, Chen, & Xiang, 2019). Beyond disease treatment, a simpler example of menstrual blood derived-stem cells used in repair is found in treating cutaneous wounds, which are injuries to the skin. The goal of cutaneous wound repair is to restore the damaged tissue to its original state, leaving no scars typically found after an area is damaged. Yanling Zhang et al. found that after mice were injected with menstrual blood-derived stem cells, they exhibited significantly improved wound healing and enhanced new blood vessel formation at the injury site (2016). Beyond generating specific cells used in the rebuilding of damaged tissue, menstrual blood-derived stem cells also secreted several proteins used in cell signaling for wound repair post-injection, increasing their ability to heal wounds to their original state (Zhang et al., 2016).
While researchers are optimistic, there remains a degree of uncertainty in the efficacy of menstrual blood-derived stem cells. Recent research has reported that menstrual blood-derived stem cells from donors over the age of 40 showed a diminished long-term proliferation capacity, raising concerns surrounding the efficacy among different age groups of donors (Haining Lv, Hu, Cui, & Jia, 2018). Ongoing clinical studies continue to evaluate the safety and efficacy of menstrual blood-derived stem cells, and research will continue to reveal what may be in store for its future as a possible restorative tool.
In the complex world of regenerative medicine, the potential of menstrual blood in stem-cell therapy remains yet to be fully discovered, but ongoing research offers hope in the next “bloody” breakthrough.
References
Chen, L., Qu, J., Cheng, T., Chen, X., & Xiang, C. (2019). Menstrual blood-derived stem cells: toward therapeutic mechanisms, novel strategies, and future perspectives in the treatment of diseases. 10(1). https://doi.org/10.1186/s13287-019-1503-7
Chen, L., Qu, J., & Xiang, C. (2019). The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. 10(1). https://doi.org/10.1186/s13287-018-1105-9
Faramarzi, H., Mehrabani, D., Fard, M., Akhavan, M., Zare, S., Bakhshalizadeh, S., … Shirazi, R. (2016). The Potential of Menstrual Blood-Derived Stem Cells in Differentiation to Epidermal Lineage: A Preliminary Report. World Journal of Plastic Surgery, 5(1), 26–31. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4904135/
Haining Lv, Hu, Y., Cui, Z., & Jia, H. (2018). Human menstrual blood: a renewable and sustainable source of stem cells for regenerative medicine. 9(1). https://doi.org/10.1186/s13287-018-1067-y
Rohatgi, A., & Dash, S. (2023). Period poverty and mental health of menstruators during COVID-19 pandemic: Lessons and implications for the future. Frontiers in Global Women’s Health, 4. https://doi.org/10.3389/fgwh.2023.1128169
Sayeh Khanjani, Mohammadreza Khanmohammadi, Amir-Hassan Zarnani, Mohammad Mehdi Akhondi, Ahani, A., Zahra Ghaempanah, … Somaieh Kazemnejad. (2014). Comparative Evaluation of Differentiation Potential of Menstrual Blood- Versus Bone Marrow- Derived Stem Cells into Hepatocyte-Like Cells. 9(2), e86075–e86075. https://doi.org/10.1371/journal.pone.0086075
Zhang, Y., Lin, X., Dai, Y., Hu, X., Zhu, H., Jiang, Y., & Zhang, S. (2016). Endometrial stem cells repair injured endometrium and induce angiogenesis via AKT and ERK pathways. 152(5), 389–402. https://doi.org/10.1530/rep-16-0286
