For some, February 14th means sweet red roses, heart-shaped chocolates, and chubby teddy bears. For others, February 14th holds a totally different meaning; it’s a celebration of surviving complex surgeries, cramped hospital beds, and the gift of life. While February 14th is notably known as Valentine's Day, it is also World Congenital Heart Disease (CHD) Awareness Day for the 2.4 million people (including Miley Cyrus, Tedy Bruschi, Jessie J, and several others) who live with congenital heart disease. CHD affects 1 in 4 live births (Center for Disease Control and Prevention, 2023). Yet, there is no system to track the birth defect beyond early childhood.
The prevalence of CHD reflects the highly complex nature of heart development: there must be precise coordination of cardiac cell specification, morphology, and differentiation to guide the development of the heart’s structure in just 3 weeks in humans and 1 week in mice. The heart begins as a linear tube, subsequently undergoing cardiac looping, which is critical for the orientation of the heart chambers. This is followed by the maturation of other heart structures like the chambers, atrial, intraventricular septation, and valves (Edwards, 2023). The transition from a simple linear heart tube to a four-chambered heart must be precise—any minor discrepancy can lead to life-threatening cardiac diseases.
Because genetic mutations are associated with an increased risk of birth defects, most research on CHD has primarily focused on gene expression. However, the potential implications of assessing factors like cardiac proteins, metabolic pathways, and even stem cells in relation to CHD are vast and could provide valuable insights into therapeutic solutions and the cellular/molecular intricacies of cardiovascular diseases altogether.
Recently, researchers at the University of North Carolina Chapel School of Medicine discovered the thousands of expressed cardiac proteins found during critical stages of embryonic development. Using protein labeling techniques, researchers were able to identify and measure the abundance of proteins from heart tissue samples as well as identify the molecular pathways that were activated during specific stages of heart development (UNC Health, 2023). A key finding points to the proteins highly expressed during the midgestation period of heart development from the mevalonate (MVA) pathway. The MVA pathway regulates embryonic cardiomyocyte (embryonic heart cells) cycling and signaling, and guards cells from accumulation of toxic end products (Guerra, 2021). A loss of an MVA pathway enzyme called geranylgeranyl pyrophosphate synthase plays a critical role in the maturation of the heart’s ventricular chamber, resulting in embryonic death (Phillips, 2018). A thorough investigation of the MVA pathway can provide valuable insights into the underlying causes of congenital heart diseases.
While studying the MVA pathway may potentially give researchers a better understanding of CHD, it may not be an actionable solution to actually cure CHD. Therapeutic proteins tend to have high activity and specificity, but their short half-lives and low solubility serve as a barrier to prolonging their therapeutic activities (Bhawani, 2018). Thankfully, the use of stem cells has been a medical breakthrough with great therapeutic and biotechnological potential. Stem cells can replace damaged or dysfunctional cells, deliver therapeutic proteins after they have been engineered to do so, and differentiate into specialized cell types (Center for Disease and Prevention, 2023). Additionally, most stem cells are able to renew and replace themselves, such as blood-forming stem cells and skin-forming cells, which are replaced periodically for everyday health. However, neurons and cardiomyocytes (heart cells) for example, are cells that don’t participate in tissue renewal or replacement, especially as cells mature into adulthood (Weiss, 2013). This makes it even more difficult to treat neurological and cardiovascular diseases like CHD.
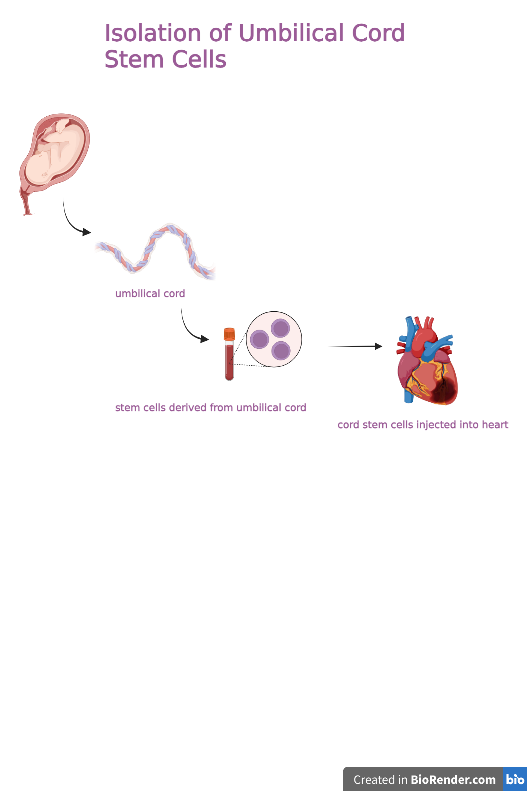
Lately, scientists have identified the role of umbilical cord stem cells (UMSC) that not only combat the ethical and scientific issues surrounding embryonic stem cell research, but are multipotent, readily available, and inexpensive sources capable of forming several cell types. During birth, umbilical cords can be collected, and the cells extracted from the blood, placenta, amniotic fluid, and surrounding tissues can be cryogenically stored for decades. These cells can then be injected into the heart for therapeutic use as needed (Weiss, 2013).
Since the first successful umbilical cord blood transplant in 1988, umbilical cord blood has been proven to have therapeutic value for patients with bone-marrow related deficiencies, genetic diseases, inborn errors of metabolism, and more recently, congenital heart diseases (Weiss, 2013). Using in-vitro differentiation methods, Wang et. al concluded that UMSCs can be induced to exhibit cardiomyocyte morphology, produce cardiac muscle proteins, and they demonstrate excellent cell growth properties for human modeled cardiovascular disease engineering (Reyhani, 2020). Since most CHD related diseases are rooted in structural faults, UMSCs can potentially promote full development of specific structures, account for missing proteins essential for development and reverse the anatomical causes of CHDs. The accessible nature of umbilical cords right after birth makes this approach more realistic and could even override the daunting need for heart transplants in the future if implemented effectively.
These new approaches to combating CHDs have shown great promise, laying the groundwork for an eventual cure in the years to come. Studying the molecular pathways involved in heart development will bring us closer to understanding CHDs, and umbilical cord cell therapy will provide us with a strategic plan to cure it. As with any newly discovered therapeutics, additional research on UMSC and clinical trials will prove its advantageous qualities. What was once regarded as tissue thrown into biohazardous waste bags will be studied closely and used for its therapeutic, life-saving properties in the future.
References
Bhawani, S., et al. Polymer Based Protein Therapeutics. (2018). Polymer Based Protein Therapeutics.
Buijtendijk, M., Barnett, P., van den Hoff, M. Development of the human heart. American Journal of Medical Genetics. (2020) Development of the human heart - PMC
Edwards, W., et al. Quantitative proteomic profiling identifies global protein network dynamics in murine embryonic heart development. Developmental Cell. (2023). https://www.sciencedirect.com/science/article/pii/S1534580723001818?via%3Dihub
Ghafarzadeh, M., Namdari, M., Eatemadi, A. Stem Cell Therapies for Congenital Heart Disease. (2016). https://www.sciencedirect.com/science/article/pii/S0753332216317942?via%3Dihub
Guerra, B., et al. The Mevalonate Pathway, a Metabolic Target in Cancer Therapy. Frontiers. (2021) The Mevalonate Pathway, a Metabolic Target in Cancer Therapy
Phillips, H. Protein Geranylgeranylation: A Possible New Player in Congenital Heart Defects. Cardiovascular Research. (2018). Protein geranylgeranylation: a possible new player in congenital heart defects | Cardiovascular Research | Oxford Academic
Researchers Target Protein Pathways Behind Congenital Heart Disease. UNC Health. (2023). Researchers Target Proteins, Pathways Behind Congenital Heart Disease | Newsroom
Reyhani, S., Abbaspanah, B., Mousavi, S., Umbilical cord-derived mesenchymal stem cells in neurodegenerative disorders: from literature to clinical practice. Future Medicine. (2020). Umbilical cord-derived mesenchymal stem cells in neurodegenerative disorders: from literature to clinical practice
Umbilical Cord Blood Banking. The American College of Obstetricians and Gynecologists. (2015). Umbilical Cord Blood Banking | ACOG
Weiss, M., Troyer, D. Stem Cells in the Umbilical Cord. (2013). Stem Cells in the Umbilical Cord - PMC
What are congenital heart defects?. Centers for Disease Control and Prevention. (2023). What are Congenital Heart Defects? | CDC)
