Ask a person what they remember from biology, and they will likely say mitochondria. Known as a powerhouse of the cell, a mitochondrion is an organelle, or a cell component, that converts glucose into adenosine triphosphate (ATP), the molecular energy that keeps our cells—liver, heart, brain, and more—functioning. A mitochondrion is also a unique organelle because it is the only one with its own DNA that codes specific proteins for ATP synthesis, cell communication, calcium signaling, and cell death (Chan, 2006). When these critical and complicated processes operate normally, our cells can carry out their jobs basic to human survival. But when something goes awry, mitochondria, in personified terms, can get stressed out.
During ATP synthesis, a small percentage of oxygen molecules are converted into free radicals, or reactive oxygen species (ROS) (Pizzino et al., 2017). When cells are unable to detoxify these unstable molecules via antioxidants, the mitochondria undergo oxidative stress. High levels of ROS can damage lipids, proteins, RNA, and DNA, leading to effects in cancer, diabetes, Alzheimer’s disease, schizophrenia, and, more recently discovered, anxiety (Pizzino et al., 2017). In the past decade, scientists have begun studying mitochondria in relation to anxiety-related disorders based on two main lines of reasoning. The first being that the brain is only 2% of human mass, but nearly a quarter of the body’s oxygen and glucose is dedicated to ATP respiration for the millions of neurons across brain regions (Misgeld & Schwartz, 2017). And the second being that most adult neurons do not replicate or undergo cell death, making these cells more vulnerable to mitochondrial DNA (mtDNA) mutations and oxidative stress (Fedoce et al., 2018). Thus, a growing number of scientists believe that abnormalities in mitochondria and the ATP process may be an underlying cause of anxiety.
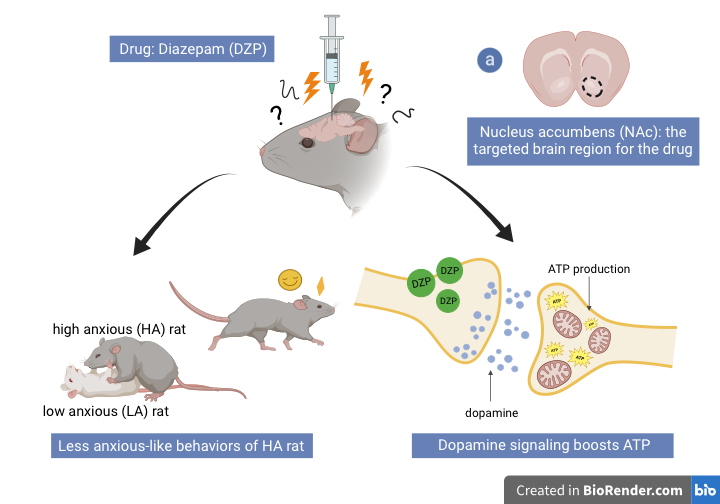
To further understand this, in a study on rats, chronic stress led to increases in mtDNA mutations and mitophagy, the elimination of mitochondria, in the amygdala (Duan et al., 2021). Because neurons require energy to communicate with one another, these findings suggest that a consistent loss of mitochondria may impair synaptic signaling within the amygdala, the center of emotional processing. In another study comparing high-anxious (HA) and low-anxious (LA) rats, high-anxious rats exhibited decreased ATP, but increased ROS production in the nucleus accumbens, the brain region involved in social reward and motivation for rats and humans (Hollis et al., 2015). These mitochondrial changes were reflected in the behaviors of HA rats; compared to their LA counterparts, the rodents displayed less effort and motivation when solving a maze, exploring a new object in their cage, and swimming. A follow-up experiment on the high-anxious rats revealed that Benzodiazepines, a class of anxiety-related drugs, boosted ATP production and linked mitochondria to dopamine signaling in the nucleus accumbens (van der Kooij et al., 2018). The researchers discovered that the activated dopamine receptors increased ATP synthesis, improving the behaviors of the high-anxious rats (van der Kooij et al., 2018).
Clinical studies on humans have enhanced these animal model findings. Patients with mitochondrial disorders arising from deletions in the mtDNA have comorbidities with mood disorders and experience symptoms like phobias and panic attacks (Filiou & Sandi, 2019). A study done on children with oxidative phosphorylation disorders, a type of mitochondrial disease, found that they displayed significantly more depressive behavior compared to healthy controls (Morava et al., 2010). Another study confirmed that 70% of the participants diagnosed with mitochondrial dysfunction met the criteria for mental illness (Fattal et al., 2006). Moreover, researchers analyzed the relationship between mitochondrial DNA and C-reactive protein (CRP), a neuroinflammatory marker associated with anxiety and depression, and found 24 significant interactions between mitochondria and this protein (Liu et al., 2023). ROS is also involved in inflammation, so these results support mitochondria’s importance in anxiety and depression (Fabisiak et al., 2022).
Together, the preliminary findings suggest that new medication targeted at mitochondria can treat anxiety more effectively than can medication that targets just the symptoms (Nussbaumer et al., 2016). Whether improving well-established drugs like Benzodiazepines, novel drugs like Reelin (Brymer et al., 2020), or implementing lifestyle changes in fitness and diet (Memme et al., 2019; Ülgen et al., 2023), the mitochondrion has shown itself to be not only the powerhouse of the cell, but also the potential driver of a healthy and stress-free brain.
References
Brymer, K.J., Johnston, J., Botterill, J.J., Romay-Tallon, R., Mitchell, M.A., Allen, J., Pinna, G., Caruncho, H.J., Kalynchuk, L.E. (2020). Fast-acting antiFast acting antidepressant-like effects of Reelin evaluated in the repeated corticosterone chronic stress paradigm. Neuropsychopharmacology, 45, 1707-1716. doi: 10.1038/s41386-020-0609-z.
Chan, D.C. (2006). Mitochondria: Dynamic organelles in disease, aging, and development. Cell, 125(7), 1241-1242. doi: 10.1016/j.cell.2006.06.010.
Duan, K., Gu, Q., Petralia, R.S., Wang, Y., Panja, D., Liu, X., Lehmann, M.L., Zhu, H., Zhu, J., Li, Zheng. (2021). Mitophagy in the basolateral amygdala mediates increased anxiety induced by aversive social experience. Neuron, 109(23), 3793–3809. doi: 10.1016/j.neuron.2021.09.008.
Fabisiak, T. & Patel, M. (2022). Cross talk between neuroinflammation and oxidative stress in epilepsy. Frontiers in Cell and Developmental Biology, 10:976953, doi: 10.3389/fcell.2022.976953.
Fattal, O., Link, J., Quinn, K., Cohen, B.H., Franco, K. (2007). Psychiatric comorbidity in 36 adults with mitochondrial cytopathies. CNS Spectrums, 12(), 429-438. doi: 10.1017/S1092852900015303.
Fedoce, A. D. G., Ferreira, F., Bota, R. G., Bonet-Costa, V., Sun, P. Y., Davies, K. J. A. (2018). The role of oxidative stress in anxiety disorder: Cause or consequence?. Free radical research, 52(7), 737–750. doi: 10.1080/10715762.2018.1475733.
Filiou, M.D. & Sandi, C. (2019). Anxiety and brain mitochondria: A bidirectional crosstalk. Trends in Neuroscience, 42(9), 573-588. doi: 10.1016/j.tins.2019.07.002.
Hollis, F., van der Kooij, M.A., Zanoletti, O., Lozano, L., Canto C., Sandi, C. (2015). Mitochondrial function in the brain like anxiety with social subordination. Proceedings of the National Academy of Sciences of the United States of America, 12(50), 15496-91. doi: 10.1073/pnas.1512653112.
Liu,, L., Cheng, S., Qi, X., MENG, p., Yang, X., Pan, C., Chen, Y., Zhang, H., Zhang, Z., Zhang, J., i, C., Wen, Y., Jia, Y., Cheng, B., Zhang, F. (2023). Mitochondria-wide association study observed significant interactions of mitochondrial respiratory and the inflammatory in the development of anxiety and depression. Translational Psychiatry 13, 216. doi: 10.1038/s41398-023-02518-y.
Memme, J.M., Erlich, A.T., Phukan, G., Hood, D.A. (2019). Exercise and mitochondrial health. The Journal of Physiology, 599(3), 803-817. doi: 10.113/JP278853.
Misgeld, T., & Schwarz, T. L. (2017). Mitostasis in neurons: Maintaining mitochondria in an extended cellular architecture. Neuron, 96(3), 651–666. doi: 10.1016/j.neuron.2017.09.055.
Morava, E., Gardeitchik, T., Kozicz, T., de Boer, L.D., Koene, S., de Vries, M.C., Roobl, T., Rodenburg, R.J.T., Verhaak, C.M. (2010). Depressive behavior in children diagnosed with a mitochondrial disorder. Mitochondrion, 10(5), doi: 10.1016/j.mito.2010.05.011.
Nussbaumer, M., Asara, J.M., Teplystska, L., Murphy, M.P., Logan, A., Turck, C.W., Filiou, M.D. (2016). Selective mitochondrial targeting exerts anxiolytic effects in vivo. Neuropharmopsychology 41, 1751-1758. doi:10.1038/npp.2015.341.
Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., Squadrito, F., Altavilla, D., Bitto, A. (2017). Oxidative Stress: Harms and benefits for human health. Oxidative medicine and cellular longevity, 2017, 8416763. doi: 10.1155/2017/8416763.
Ülgen DH, Ruigrok SR, Sandi C. (2023). Powering the social brain: Mitochondria in social behaviour. Current Opinion in Neurobiology, 79, 102675. doi: 10.1016/j.conb.2022.102675.
van der Kooij, M. A., Hollis, F., Lozano, L., Zalachoras, I., Abad, S., Zanoletti, O., Grosse, J., Guillot de Suduiraut, I., Canto, C., Sandi, C. (2018). Diazepam actions in the VTA enhance social dominance and mitochondrial function in the nucleus accumbens by activation of dopamine D1 receptors. Molecular psychiatry, 23(3), 569–578. doi: 0.1038/mp.2017.135.
