Injured, immobilized, and incapacitated. Unable to even get out of bed, life becomes a battle. For too many, this is reality. As of 2023, approximately 6.7 million Americans live with a disease that destroys their self-control, memories, and fundamental identities (Alzheimer’s Association, 2023). Alzheimer’s disease wrecks both patients and families by deteriorating the patient's mental and physical abilities, and eventually causing their death. Yet, no viable cure exists, which is why understanding Alzheimer’s underlying mechanisms is crucial.
Originally discovered in 1906 by Alois Alzheimer, a clinical psychiatrist and neuroanatomist, Alzheimer’s disease was largely ignored for decades (Hippius, 2003). Later on, in the 1970s scientists began to investigate its causes and the “cholinergic hypothesis” was formed. Aptly named, it theorized that the culprit of the disease was the degeneration of cholinergic neurons, which control acetylcholine signaling (Chen et al, 2022). Then came 1991, and the “amyloid hypothesis” emerged. Amyloid beta plaques were the new targets of interest. Amyloid beta is normally a byproduct of amyloid precursor protein’s metabolism (Chen et al, 2017). In Alzheimer’s, it degrades and aggregates to form toxic plaques that induce neuronal death, causing dementia (Breijyeh and Karaman, 2020) For roughly two decades now, this theory has been the leading hypothesis in the field. Subsequently, dozens of drugs have been designed and deployed to target amyloid beta aggregation. Unfortunately, most of them have yielded little to no success (Yiannopoulou and Papageorgiou, 2020).
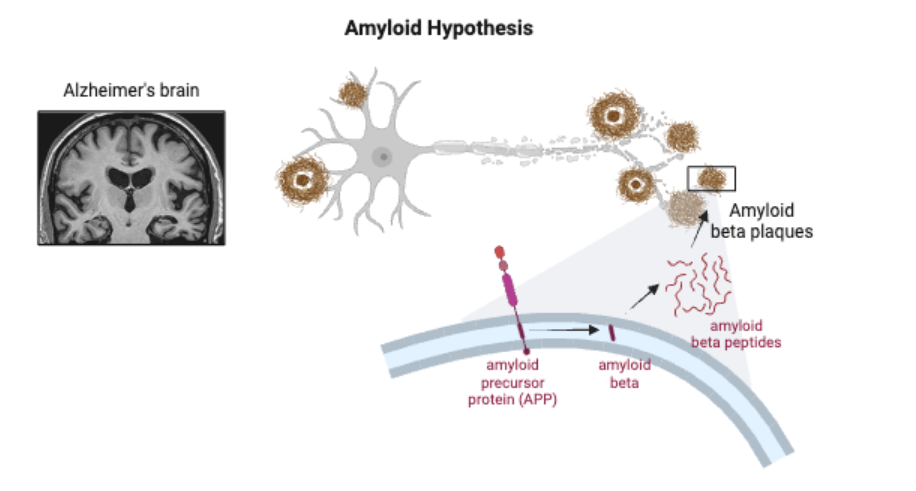
In July 2022, the scientific community faced an upheaval. A Science investigation found that a keystone 2006 Nature paper, whose first author was Sylvain Lesné, had falsified images (Piller, 2022). The paper had discovered the existence of an oligomer, or molecular complex, of amyloid beta—amyloid beta*56—and that it directly caused cognitive decline in mouse models (Lesné et al, 2006). The paper had been cited in 2300 other scholarly articles and single-handedly increased funding for amyloid studies by hundreds of millions of dollars (Piller, 2022). Unfortunately, the Science investigation didn’t just identify image fabrications in this one paper. More than 20 of Lesné’s papers were flagged, and the original 2006 keystone paper was eventually retracted. The amyloid hypothesis was slowly unraveling.
Of course, the investigation does not disprove the amyloid hypothesis. There are direct correlations between amyloid beta plaques and degeneration, and researchers’ overall consensus is that it still stands (Briejyeh and Karaman, 2020). However, it does change what mechanisms treatments should target. Instead of focusing solely on amyloid beta, the drug pipeline should consider a more holistic view of the causes of Alzheimers’—proteins like tau and oxidative stress, for instance (Ratan et al, 2023). More importantly, though, the image fabrications are a testament to the serious consequences of violating academic integrity.
Violations of academic integrity occur far too often. In fact, it is estimated that 2.9% of scientists have committed at least one act of falsification, fabrication, or plagiarism (Xie et al, 2021). This case is just one example, and the scientific community must learn to prioritize integrity. One paper with falsified images has the potential to become a red herring, perhaps leading to hundreds of millions of dollars and time being funded towards the wrong targets. Diseases like Alzheimer’s have incidence rates that are only increasing (Alzheimer’s Association, 2023), and patients need efficient and innovative treatments—starting from researching a holistic view and conducting science with integrity.
References
Alzheimer’s Association. 2023 Alzheimer’s disease facts and figures. (2023). Alzheimer’s & Dementia, 19(4), 1598–1695. https://doi.org/10.1002/alz.13016
Breijyeh, Z., & Karaman, R. (2020). Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules, 25(24). https://doi.org/10.3390/molecules25245789
Chen, G., Xu, T., Yan, Y., Zhou, Y., Jiang, Y., Melcher, K., & Xu, H. E. (2017). Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacologica Sinica, 38(9), 1205–1235. https://doi.org/10.1038/aps.2017.28
Chen, Z.-R., Huang, J.-B., Yang, S.-L., & Hong, F.-F. (2022). Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules, 27(6). https://doi.org/10.3390/molecules27061816
Hippius, H., & Neundörfer, G. (2003). The discovery of Alzheimer’s disease. Dialogues in Clinical Neuroscience, 5(1), 101–108.
Lesné, S., Koh, M. T., Kotilinek, L., Kayed, R., Glabe, C. G., Yang, A., Gallagher, M., & Ashe, K. H. (2006). RETRACTED ARTICLE: A specific amyloid-β protein assembly in the brain impairs memory. Nature, 440(7082), 352–357. https://doi.org/10.1038/nature04533
Piller, C. (2022). Blots on a field? Science, 377(6604), 358–363. https://doi.org/10.1126/science.ade0209
Ratan, Y., Rajput, A., Maleysm, S., Pareek, A., Jain, V., Pareek, A., Kaur, R., & Singh, G. (2023). An Insight into Cellular and Molecular Mechanisms Underlying the Pathogenesis of Neurodegeneration in Alzheimer’s Disease. Biomedicines, 11(5), Article 5. https://doi.org/10.3390/biomedicines11051398
Xie, Y., Wang, K., & Kong, Y. (2021). Prevalence of Research Misconduct and Questionable Research Practices: A Systematic Review and Meta-Analysis. Science and Engineering Ethics, 27(4), 41. https://doi.org/10.1007/s11948-021-00314-9
Yiannopoulou, K. G., & Papageorgiou, S. G. (2020). Current and Future Treatments in Alzheimer Disease: An Update. Journal of Central Nervous System Disease, 12, 1179573520907397. https://doi.org/10.1177/1179573520907397
