Carbon monoxide’s lethal reputation as a “silent killer” is well-deserved. From turning on a car engine in a closed garage to breathing in smoke during a fire, this colorless, odorless, and tasteless gas can unsuspectingly kill an adult human within minutes (Blumenthal, 2001). Despite its deadly nature, emerging research suggests that low doses of the chemical could actually have therapeutic benefits for multiple illnesses, particularly cardiovascular disease and cancer.
What is carbon monoxide?
Carbon monoxide is made of one carbon bonded to oxygen, with the chemical formula CO. At high concentrations, CO interferes with oxygen delivery to vital tissues and organs in the body. This occurs because CO has 210 times the affinity for hemoglobin, the protein in red blood cells responsible for oxygen uptake and transport, so it easily displaces oxygen in the blood, leading to oxygen deprivation and death (Blumenthal, 2001). At lower concentrations, CO can also result in extremely serious symptoms, including headaches, vomiting, lethargy, and dizziness if exposed long-term (Blumenthal, 2001).
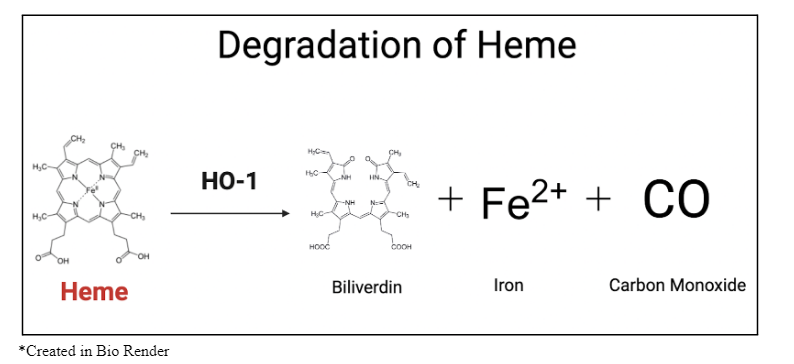
Although acute and chronic exposure to CO in the ambient environment can harm humans, CO is naturally produced in small quantities within the body and serves various purposes to support biological processes. CO is made as a byproduct during the degradation of heme, the iron-containing component of hemoglobin in the blood, by the enzyme heme oxygenase (HO-1) (Chatterjee, 2004). It then binds to other heme-containing proteins, functioning as a neurotransmitter and exhibiting anti-inflammatory and antioxidant effects (Chatterjee, 2004; Marazioti et al 2011). Understanding the role of CO produced within the body has led researchers to further explore its medical potential as a therapeutic, rather than poisonous, agent.
CO and Cardiovascular Disease
CO is known to have many adverse effects on heart health, from arrhythmias to congestive heart failure (lee et al., 2015). However, several animal studies show that low doses of CO induce blood vessel relaxation, improving conditions like hypertension (high blood pressure) and preventing the excessive narrowing of blood vessels in the heart (Marazioti et al 2011) . Additionally, CO can weakly activate soluble guanylyl cyclase, a protein crucial in decreasing smooth muscle tone and preventing platelet accumulation and adhesion of white blood cells to the vessel wall (Marazioti et al 2011). These traits indicate that CO could serve as a preventative treatment for many cardiovascular diseases, including atherosclerotic cardiovascular disease, coronary artery disease, and heart attacks . Since heart disease is the leading cause of death globally, CO provides a promising avenue for reducing morbidity and mortality associated with these conditions.
CO and Cancer
Like cardiovascular disease, cancer is a leading cause of death worldwide, with nearly 10 million deaths annually (“Global cancer burden,” 2024). Although it varies in type and can have numerous underlying factors, cancer commonly involves uncontrolled cell growth. Since CO plays a role in apoptosis, a type of programmed cell death, it could reduce the cancerous cell population to a manageable level [9]. Furthermore, CO blocks the proliferation of cancer cells. In a study with tumor-bearing mice, CORM treatment showed significant tumor suppression through ferroptosis, another type of programmed cell death (Cao et al., 2024). CO has even been reported to increase tumor sensitivity to chemotherapy (Cao et al., 2024). Meanwhile, in endothelial cells, the cells that line the inside of blood vessels, CO can prevent tissue injury by activating anti-apoptotic pathways [9]. However, more research is still necessary to fully understand the underlying mechanisms of these processes.
Current Problems and Future Goals
Although initial research into the medicinal use of CO has been promising, these studies consist almost exclusively of animal experiments conducted on rats, pigs, mice, and non-human primates (Motterlini & Otterbein, 2010). Consequently, more human research is required before CO-based treatments can move forward towards clinical use.
Another issue that requires further investigation is the route of delivery. Inhalation may seem like the most intuitive way to administer CO, but this mode of delivery makes it difficult to control dosage, increasing the risk of CO poisoning (Motterlini & Otterbein, 2010). To address this concern, researchers in the Motterlini lab developed Carbon Monoxide Releasing Molecules (CORMs), which are compounds capable of releasing controlled amounts of CO within the body (Chu et al., 2021). They contain transition metals like iron to bind CO, as CO has strong affinity for metal compounds. CORMs have been used in many animal studies and have played a significant role in advancing understanding of CO’s therapeutic benefits. However, recent discussion has called into question whether some of the observed biological effects can be attributed solely to CO, since the metals may be able to react with proteins and facilitate chemical reactions (Wang et al., 2020). Additionally, concerns about the heavy metal toxicity make CORMs a less-than-ideal solution (Wang et al., 2020). Developing a dose-controlled route of administration, orally or intravenously, is still a future hurdle to clear.
Despite the obstacles, the pharmaceutical potential of carbon monoxide represents a transformative development in medicine. As research advances, this previously abhorred compound could become a pivotal component in developing novel treatments, improving patient outcomes, and expanding the horizons of modern medicine.
References
- Blumenthal, I. (2001). Carbon monoxide poisoning. Journal of the royal society of medicine, 94(6),
270-272.
- Cao, W., Sun, M., Yu, K. N., Zhao, L., Feng, Y., Tan, C., ... & Han, W. (2024). Exogenous carbon
monoxide promotes GPX4-dependent ferroptosis through ROS/GSK3β axis in non-small cell lung
cancer. Cell Death Discovery, 10(1), 42.
- Chatterjee, P. K. (2004). Water‐soluble carbon monoxide‐releasing molecules: helping to elucidate the
vascular activity of the ‘silent killer’. British journal of pharmacology, 142(3), 391-393.
- Chu, L. M., Shaefi, S., Byrne, J. D., de Souza, R. W. A., & Otterbein, L. E. (2021). Carbon monoxide and
a change of heart. Redox biology, 48, 102183.
- Durante, W., Johnson, F. K., & Johnson, R. A. (2006). Role of carbon monxide in cardiovascular function.
Journal of cellular and molecular medicine, 10(3), 672-686.
- Global cancer burden growing, amidst mounting need for services. (2024, February 1). World Health
Organization . Retrieved June 24, 2024, from https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services.
- Lee, F. Y., Chen, W. K., Lin, C. L., & Kao, C. H. (2015). Carbon monoxide poisoning and subsequent
cardiovascular disease risk: a nationwide population-based cohort study. Medicine, 94(10), e624.
- Marazioti, A., Bucci, M., Coletta, C., Vellecco, V., Baskaran, P., Szabó, C., ... & Papapetropoulos, A.
(2011). Inhibition of nitric oxide–stimulated vasorelaxation by carbon monoxide-releasing
molecules. Arteriosclerosis, thrombosis, and vascular biology, 31(11), 2570-2576.
- Motterlini, R., & Otterbein, L. E. (2010). The therapeutic potential of carbon monoxide. Nature reviews
Drug discovery, 9(9), 728-743.
- Wang, M., Yang, X., Pan, Z., Wang, Y., De La Cruz, L. K., Wang, B., & Tan, C. (2020). Towards “CO in a
pill”: Pharmacokinetic studies of carbon monoxide prodrugs in mice. Journal of Controlled
Release, 327, 174-185.
