Performing vertebroplasty with millimeter precision is a bit like repairing the foundation of a house through a keyhole, except the house is someone’s spine, and the material you’re injecting hardens within minutes. A slight misalignment or deviation can cause bone cement to leak into nerves or blood vessels, turning a pain-relieving procedure into a source of irreversible damage. In such high-stakes conditions, robotic systems are no longer futuristic novelties—they’re becoming indispensable allies in the operating room.
Vertebral compression fractures (VCFs), most often caused by osteoporosis, are common and debilitating. When vertebrae collapse under the pressure of weakened bone, patients experience intense back pain, reduced mobility, and progressive spinal deformity. The standard treatment for VCFs is percutaneous vertebroplasty (PVP), a minimally invasive procedure in which a surgeon inserts a thin, needle-like instrument (known as a trocar) through the skin and into the fractured vertebra. Once inside, polymethylmethacrylate (PMMA), a medical-grade bone cement, is injected into the fracture (Medical Advisory Secretariat, 2010). The cement hardens within minutes, acting as an internal cast that stabilizes the bone, alleviates pain, and restores structure. 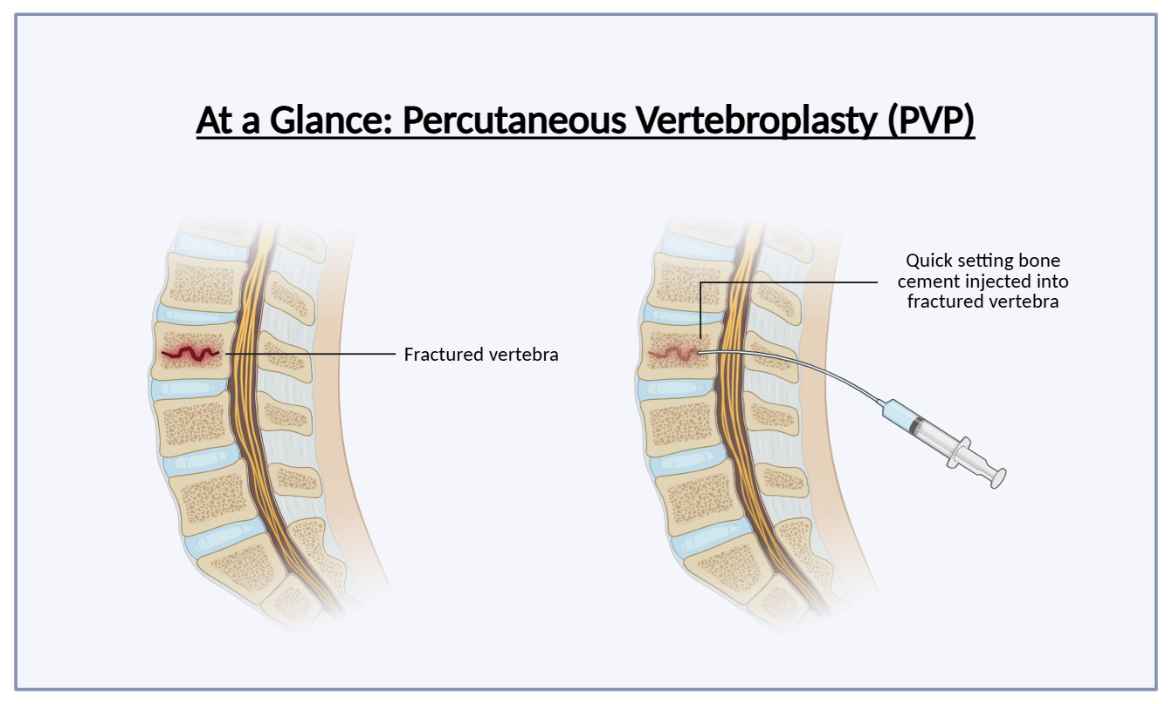
Traditionally, this has been guided by fluoroscopy, a real-time X-ray imaging technique. But fluoroscopy has its drawbacks: it requires repeated radiation exposure, relies heavily on the surgeon’s hand-eye coordination, and often results in uneven cement distribution or leakage (Vanzant & Mukhdomi, 2021). This is not just a technical issue—it is a safety risk. Improper cement placement can lead to pulmonary embolisms, nerve damage, or adjacent-level fractures.
Robotic-assisted vertebroplasty offers a compelling upgrade. Systems like TiRobot combine preoperative CT scans with advanced software-guided navigation to calculate optimal needle trajectories based on patient-specific anatomy (Wang et al., 2021). These platforms enable sub-millimeter precision, guiding the trocar through complex bony pathways with consistent accuracy.
In a randomized clinical trial of 96 patients with osteoporotic vertebral compression fractures (OVCFs), those who received robotic-assisted PVP showed significantly greater cement dispersion, higher injection volumes, and lower rates of leakage compared to those treated with conventional fluoroscopy-guided techniques. Notably, the robotic group experiences fewer fluoroscopy exposures, meaning less cumulative radiation (Zhu et al., 2025).
A meta-analysis and validation cohort comprising of 385 patients undergoing PVP or kyphoplasty (a related spine augmentation technique) further reinforced these findings. Robotic-assisted procedures were associated with significantly fewer cement leakages and follow-up fractures. The study also introduced nomograms, or graphical risk prediction tools, that help personalize treatment by identifying patients most likely to benefit from robotic support based on variables like bone density, fracture angle, and surgical approach (Li et al., 2024).
But beyond the data lies a broader shift in surgical philosophy. The promise of robotic assistance is not just about technical accuracy–it’s about standardization. Manual procedures vary by institution, by surgeon, and by fatigue. Robotics introduced reproducibility in a domain where inconsistency can cause harm.
Yet adoption is far from universal. Critics of robotic-assisted vertebroplasty are quick to cite high costs, technical complexity, and the steep learning curve required to implement these systems effectively (Antonacci et al., 2024). These are valid concerns. Hospitals must weigh upfront investment against downstream benefits, which often don’t materialize until long after the robot is wheeled into the OR. But ignoring robotics on these grounds risks widening an already troubling gap in surgical care.
The conversations should not stop at what’s possible—it must also ask: who gets access?
Academic hospitals and private institutions are early adopters, but what about rural centers or community clinics treating aging populations with limited resources? If robotic PVP truly reduces complications and improves safety, then relegating it to elite settings risks turning surgical precision into a privilege.
At the same time, to dismiss robotics as excessive misses the point. Across specialities, from neurosurgery to urology, we have seen technology transform the field, not by replacing surgeons, but by enhancing them. Robotics doesn’t remove judgment; it refines execution. It allows surgeons to focus on decision-making while delegating precision to the tools best equipped for it.
Moreover, innovations like AI-generated path planning, real-time haptic feedback, and augmented reality overlays are already on the horizon, making robotic systems more intuitive and adaptable. As these tools evolve, we are likely to see reductions in setup time, cost, and training barriers—much like we did with laparoscopic surgery, which was once novel and is now standard.
Robotic-assisted vertebroplasty represents more than just a technical upgrade—it’s a paradigm shift. With growing evidence that it reduces cement leakage, lowers refracture risk, improves cement spread, and reduces radiation exposure, this approach deserves recognition not as an emerging novelty but as a possible new gold standard in spinal care. Hospitals and surgical centers aiming to lead in minimally invasive spine surgery should prioritize adopting robotic systems, investing in training, and funding long-term research. The spine may be delicate, but our standards for treating it shouldn’t be.
References
- Medical Advisory Secretariat (2010). Percutaneous vertebroplasty for treatment of painful osteoporotic vertebral compression fractures: an evidence-based analysis. Ontario health technology assessment series, 10(19), 1–45.
- Vanzant, D., & Mukhdomi, J. (2023). Safety of Fluoroscopy in Patient, Operator, and Technician. In StatPearls. StatPearls Publishing.
- Wang, B., Cao, J., Chang, J., Yin, G., Cai, W., Li, Q., Huang, Z., Yu, L., & Cao, X. (2021). Effectiveness of Tirobot-assisted vertebroplasty in treating thoracolumbar osteoporotic compression fracture. Journal of orthopaedic surgery and research, 16(1), 65. https://doi.org/10.1186/s13018-021-02211-0
- Zhu, Y., Chen, H., Li, L., Yang, Y., Jia, Y., Liu, C., Xu, X., Ruan, J., Wang, B., & Liu, J. (2025). A relevant investigation of the degree of cement diffusion after robot-assisted percutaneous vertebroplasty. BMC musculoskeletal disorders, 26(1), 77. https://doi.org/10.1186/s12891-025-08315-6
- Li, H., Zou, J., & Yu, J. (2024). Effect of Robot-Assisted Surgery on Clinical Outcomes in Patients with Osteoporotic Vertebral Compression Fractures after Percutaneous Vertebral Augmentation: a Meta-Analysis and a Validation Cohort. Clinics in orthopedic surgery, 16(6), 948–961. https://doi.org/10.4055/cios24086
- Antonacci, C. L., Zeng, F., Block, A., Davey, A., & Makanji, H. (2024). Robotic-assisted spine surgery-a narrative review. Journal of spine surgery (Hong Kong), 10(2), 305–312. https://doi.org/10.21037/jss-23-40
